|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120260 |
|---|
|
Identification |
|---|
| Name: |
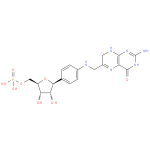
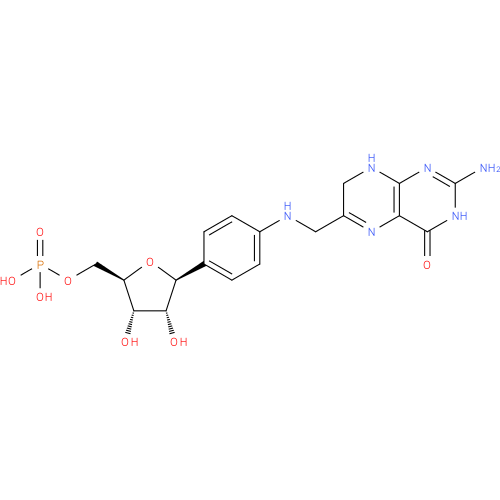
7,8-dihydropterin-6-ylmethyl-4-(β-D-ribofuranosyl)aminobenzene 5'-phosphate |
|---|
| Description: | A C-glycosyl compound that is N-[(7,8-dihydropterin-6-yl)methyl]-4-(β-D-ribofuranosyl)aniline carrying a single monophospate substituent at position 5'. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 7,8-H2pterin-6-yl-methyl-4-(β-D-ribofuranosyl)aminobenzene 5'-phosphate
|
|---|
|
Chemical Formula: |
C18H19N6O8P |
|---|
| Average Molecular Weight: |
478.357 |
|---|
| Monoisotopic Molecular
Weight: |
480.11584 |
|---|
| InChI Key: |
LTTXSGLYPYVGRL-NGFQHRJXSA-L |
|---|
| InChI: | InChI=1S/C18H21N6O8P/c19-18-23-16-12(17(27)24-18)22-10(6-21-16)5-20-9-3-1-8(2-4-9)15-14(26)13(25)11(32-15)7-31-33(28,29)30/h1-4,6,11,13-15,20,25-26H,5,7H2,(H2,28,29,30)(H3,19,21,23,24,27)/p-2/t11-,13-,14-,15+/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (1S)- 1- 1- (4- (4- {[(2- {[(2- amino- amino- 4- 4- oxo- oxo- 3,4,7,8- 3,4,7,8- tetrahydropteridin- tetrahydropteridin- 6- 6- yl)methyl]amino}phenyl)- yl)methyl]amino}phenyl)- 1,4- 1,4- anhydro- anhydro- 5- 5- O- O- phosphono- phosphono- D- D- ribitol ribitol |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | C(OP([O-])(=O)[O-])C4(C(O)C(O)C(C1(C=CC(=CC=1)NCC2(C=NC3(=C(N=2)C(N=C(N3)N)=O))))O4) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
|
Class |
Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
|
Direct Parent |
Pentose phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pentose phosphate
- Pentose-5-phosphate
- Phenolic glycoside
- Pterin
- C-glycosyl compound
- Glycosyl compound
- Monosaccharide phosphate
- Pteridine
- Phenylalkylamine
- Aniline or substituted anilines
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Aralkylamine
- Alkyl phosphate
- Benzenoid
- Monocyclic benzene moiety
- Pyrimidine
- Pyrazine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Tetrahydrofuran
- Vinylogous amide
- Heteroaromatic compound
- 1,2-diol
- Secondary alcohol
- Organoheterocyclic compound
- Secondary amine
- Dialkyl ether
- Ether
- Azacycle
- Oxacycle
- Organopnictogen compound
- Amine
- Organic nitrogen compound
- Organic oxide
- Organonitrogen compound
- Hydrocarbon derivative
- Alcohol
- Primary amine
- Organic anion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
- a small molecule (CPD-10766)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Xu H, Aurora R, Rose GD, White RH (1999)Identifying two ancient enzymes in Archaea using predicted secondary structure alignment. Nature structural biology 6, Pubmed: 10426953
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|