|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120255 |
|---|
|
Identification |
|---|
| Name: |
lotaustralin |
|---|
| Description: | Epilotaustralin is found in cereals and cereal products. Epilotaustralin is isolated from Triticum monococcum (wheat). |
|---|
|
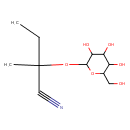
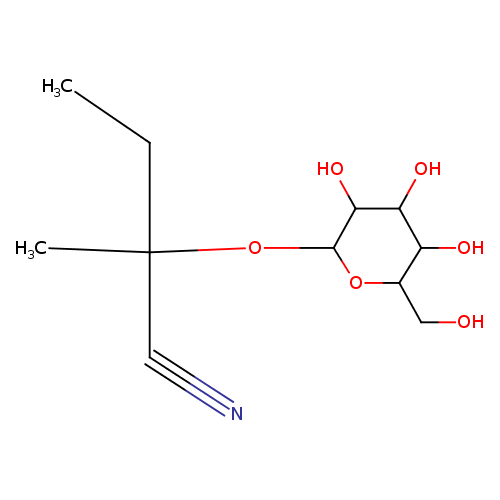
Structure |
|
|---|
| Synonyms: | - 2-hydroxy-2-methylbutyronitrile-beta-D-glucopyranoside
|
|---|
|
Chemical Formula: |
C11H19NO6 |
|---|
| Average Molecular Weight: |
261.274 |
|---|
| Monoisotopic Molecular
Weight: |
261.12125 |
|---|
| InChI Key: |
WEWBWVMTOYUPHH-UDWHLRJGSA-N |
|---|
| InChI: | InChI=1S/C11H19NO6/c1-3-11(2,5-12)18-10-9(16)8(15)7(14)6(4-13)17-10/h6-10,13-16H,3-4H2,1-2H3/t6-,7-,8+,9-,10?,11-/m1/s1 |
|---|
| CAS
number: |
534-67-8 |
|---|
| IUPAC Name: | 2-methyl-2-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}butanenitrile |
|---|
|
Traditional IUPAC Name: |
2-methyl-2-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}butanenitrile |
|---|
| SMILES: | CCC(OC1(OC(CO)C(O)C(O)C(O)1))(C#N)C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as cyanogenic glycosides. These are glycosides in which the aglycone moiety contains a cyanide group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbohydrates and carbohydrate conjugates |
|---|
| Sub Class | Glycosyl compounds |
|---|
|
Direct Parent |
Cyanogenic glycosides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Cyanogenic glycoside
- O-glycosyl compound
- Oxane
- Monosaccharide
- Secondary alcohol
- Polyol
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Nitrile
- Carbonitrile
- Acetal
- Hydrocarbon derivative
- Primary alcohol
- Organonitrogen compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
139 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 139 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|