|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120247 |
|---|
|
Identification |
|---|
| Name: |
indole-3-acetonitrile |
|---|
| Description: | A nitrile that is acetonitrile where one of the methyl hydrogens is substituted by a 1H-indol-3-yl group. |
|---|
|
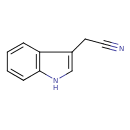
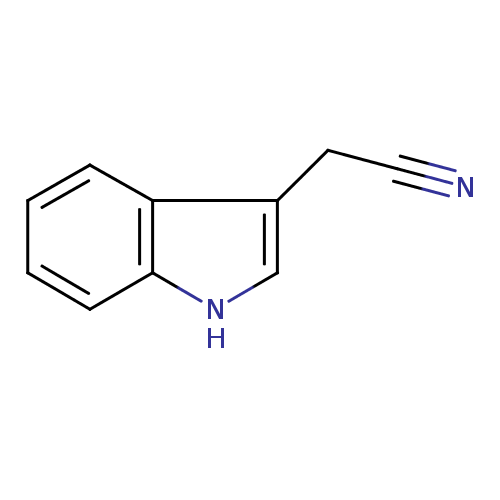
Structure |
|
|---|
| Synonyms: | - (indol-3-yl)acetonitrile
- (Indol-3-yl)acetonitrile
- (Indole-3-yl)acetonitrile
- 3-(cyanomethyl)indole
- 3-Indoleacetonitrile
- 3-indolylacetonitrile
- Indol-3-ylacetonitrile
- Indole-3-acetonitrile
|
|---|
|
Chemical Formula: |
C10H8N2 |
|---|
| Average Molecular Weight: |
156.187 |
|---|
| Monoisotopic Molecular
Weight: |
156.06874 |
|---|
| InChI Key: |
DMCPFOBLJMLSNX-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C10H8N2/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5H2 |
|---|
| CAS
number: |
771-51-7 |
|---|
| IUPAC Name: | 1H-indol-3-ylacetonitrile |
|---|
|
Traditional IUPAC Name: |
indole-3-acetonitrile |
|---|
| SMILES: | C2(NC1(C=CC=CC=1C(CC#N)=2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 3-alkylindoles. These are compounds containing an indole moiety that carries an alkyl chain at the 3-position. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Indoles and derivatives |
|---|
|
Direct Parent |
3-alkylindoles |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 3-alkylindole
- Benzenoid
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Azacycle
- Nitrile
- Carbonitrile
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
35 - 37 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 35 - 37 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- indole-3-acetate biosynthesis IV (bacteria)PWY-5025
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-004i-2950000000-640d53401ab5b06bad68 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-686ec8d86a82008f6a87 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0900000000-0f41c00c3703e04901e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-1900000000-144022b6dfec80ddd4a3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-686ec8d86a82008f6a87 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0900000000-0f41c00c3703e04901e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-1900000000-144022b6dfec80ddd4a3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-9b52919ba61c73ce0ade | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-f8b24a877c386d550c92 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-1900000000-fc82dffe3b09577e06ee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-9b52919ba61c73ce0ade | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-f8b24a877c386d550c92 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-1900000000-fc82dffe3b09577e06ee | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0a4i-2900000000-be863c7acf7363ffeedc | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Pedras MS, Nycholat CM, Montaut S, Xu Y, Khan AQ (2002)Chemical defenses of crucifers: elicitation and metabolism of phytoalexins and indole-3-acetonitrile in brown mustard and turnip. Phytochemistry 59, Pubmed: 11867093
- Michnovicz JJ, Bradlow HL (1990)Induction of estradiol metabolism by dietary indole-3-carbinol in humans. Journal of the National Cancer Institute 82, Pubmed: 2342128
|
|---|
| Synthesis Reference: |
Ahmad, A.; Spenser, Ian D. 3-Indolepyruvic acid oxime as the precursor of 3-indoleacetonitrile. Canadian Journal of Chemistry (1960), 38 1625-34. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|