|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120236 |
|---|
|
Identification |
|---|
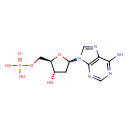
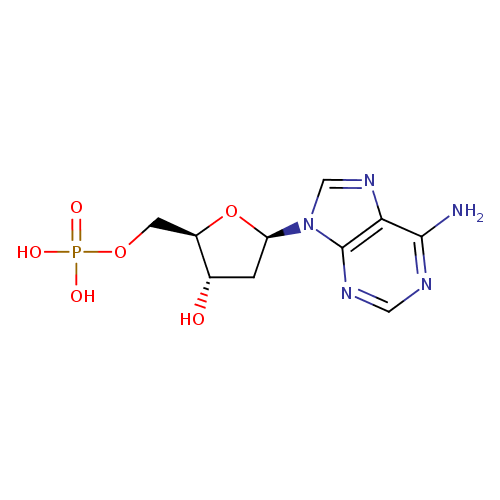
| Name: |
dAMP |
|---|
| Description: | A 2'-deoxyribonucleoside 5'-monophosphate(2−) obtained by deprotonation of the phosphate OH groups of 2'-deoxyadenosine 5'-monophosphate (dAMP). |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2'-deoxyadenosine 5'-monophosphate
- dAMP
|
|---|
|
Chemical Formula: |
C10H12N5O6P |
|---|
| Average Molecular Weight: |
329.208 |
|---|
| Monoisotopic Molecular
Weight: |
331.06818 |
|---|
| InChI Key: |
KHWCHTKSEGGWEX-RRKCRQDMSA-L |
|---|
| InChI: | InChI=1S/C10H14N5O6P/c11-9-8-10(13-3-12-9)15(4-14-8)7-1-5(16)6(21-7)2-20-22(17,18)19/h3-7,16H,1-2H2,(H2,11,12,13)(H2,17,18,19)/p-2/t5-,6+,7+/m0/s1 |
|---|
| CAS
number: |
653-63-4 |
|---|
| IUPAC Name: | 2'-deoxy-5'-O-phosphonatoadenosine |
|---|
|
Traditional IUPAC Name: |
DAMP |
|---|
| SMILES: | C(C3(C(CC(N2(C1(=C(C(=NC=N1)N)N=C2)))O3)O))OP([O-])([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine 2'-deoxyribonucleoside monophosphates. These are purine nucleotides with monophosphate group linked to the ribose moiety lacking a hydroxyl group at position 2. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine 2'-deoxyribonucleoside monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine 2'-deoxyribonucleoside monophosphate
- 6-aminopurine
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Monoalkyl phosphate
- N-substituted imidazole
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Primary aromatic amine
- Pyrimidine
- Alkyl phosphate
- Imidolactam
- Imidazole
- Heteroaromatic compound
- Azole
- Oxolane
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organonitrogen compound
- Alcohol
- Organic nitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Amine
- Primary amine
- Organic oxygen compound
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
148 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 148 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (6 TMS) | splash10-001i-9210000000-52089369f69d88f5ea1c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0089-4096000000-d903c4b1613b2ac5f708 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-001i-9010000000-c50cec0a2ca71d42d524 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-001i-9300000000-8ebf385d33982fcaef9f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-001i-0209000000-05ded3b31c4db3d6c016 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive | splash10-0019-1907000000-50567fa8c2638c9e2b6f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-003r-9804000000-9196bd87057cc65827a3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Hohenester E, Hutchinson WL, Pepys MB, Wood SP: Crystal structure of a decameric complex of human serum amyloid P component with bound dAMP. J Mol Biol. 1997 Jun 20;269(4):570-8. [9217261 ]
- Avkin S, Adar S, Blander G, Livneh Z: Quantitative measurement of translesion replication in human cells: evidence for bypass of abasic sites by a replicative DNA polymerase. Proc Natl Acad Sci U S A. 2002 Mar 19;99(6):3764-9. Epub 2002 Mar 12. [11891323 ]
- Duarte V, Muller JG, Burrows CJ: Insertion of dGMP and dAMP during in vitro DNA synthesis opposite an oxidized form of 7,8-dihydro-8-oxoguanine. Nucleic Acids Res. 1999 Jan 15;27(2):496-502. [9862971 ]
- Chen XR, Li GM, Wang JR, Chen JJ: [Portal hemodynamics in patients with different syndromes of cirrhosis] Zhong Xi Yi Jie He Xue Bao. 2004 May;2(3):178-81. [15339437 ]
- Zhong H, Zang KT: Therapeutic approaches for chronic gastralgia based on differentiation of symptoms and signs. Di Yi Jun Yi Da Xue Xue Bao. 2002 Jul;22(7):639-40. [12376299 ]
- Hashimoto K, Tominaga Y, Nakabeppu Y, Moriya M: Futile short-patch DNA base excision repair of adenine:8-oxoguanine mispair. Nucleic Acids Res. 2004 Nov 5;32(19):5928-34. Print 2004. [15531653 ]
- Zhang Q, Zhang WT, Wei JJ, Wang XB, Liu P: [Combined use of factor analysis and cluster analysis in classification of traditional Chinese medical syndromes in patients with posthepatitic cirrhosis] Zhong Xi Yi Jie He Xue Bao. 2005 Jan;3(1):14-8. [15644152 ]
- Levine RL, Yang IY, Hossain M, Pandya GA, Grollman AP, Moriya M: Mutagenesis induced by a single 1,N6-ethenodeoxyadenosine adduct in human cells. Cancer Res. 2000 Aug 1;60(15):4098-104. [10945616 ]
|
|---|
| Synthesis Reference: |
Scarano, E. Incorporation of adenine-C14 into deoxyadenylic acid. Bollettino - Societa Italiana di Biologia Sperimentale (1958), 34 1620-1. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|