|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120231 |
|---|
|
Identification |
|---|
| Name: |
atrazine |
|---|
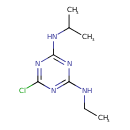
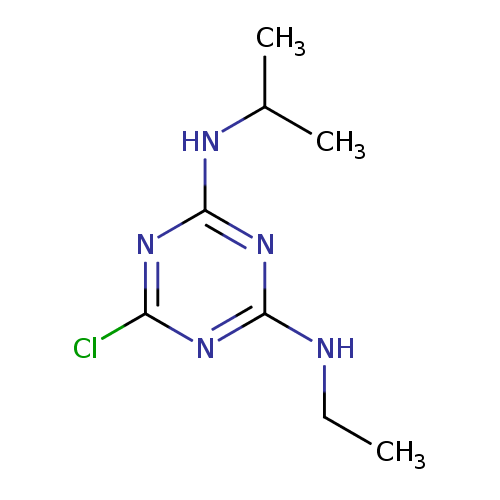
| Description: | A diamino-1,3,5-triazine that is 1,3,5-triazine-2,4-diamine substituted by a chloro group at position 6 while one of hydrogens of each amino group is replaced respectively by an ethyl and a propan-2-yl group. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-chloro-4-(ethylamino)-6-(isopropylamino)-1,3,5-triazine
- 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine
- 2-chloro-4-ethylamino-6-isopropylamino-s-triazine
- 2-CHLORO-4-ISOPROPYLAMINO-6-ETHYLAMINO-1,3,5-TRIAZINE
- 2-ethylamino-4-isopropylamino-6-chloro-s-triazine
- 6-chloro-N-ethyl-N'-(1-methylethyl)-1,3,5-triazine-2,4-diamine
- 6-chloro-N-ethyl-N'-isopropyl-1,3,5-triazine-2,4-diamine
- Atrazine
- atrazine
|
|---|
|
Chemical Formula: |
C8H14N5CL |
|---|
| Average Molecular Weight: |
215.685 |
|---|
| Monoisotopic Molecular
Weight: |
215.09378 |
|---|
| InChI Key: |
MXWJVTOOROXGIU-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C8H14ClN5/c1-4-10-7-12-6(9)13-8(14-7)11-5(2)3/h5H,4H2,1-3H3,(H2,10,11,12,13,14) |
|---|
| CAS
number: |
1912-24-9 |
|---|
| IUPAC Name: | 6-chloro-N-ethyl-N'-(propan-2-yl)-1,3,5-triazine-2,4-diamine |
|---|
|
Traditional IUPAC Name: |
atrazine |
|---|
| SMILES: | CCNC1(=NC(Cl)=NC(=N1)NC(C)C) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as chloro-s-triazines. These are aromatic compounds containing a 1,3,5-triazine ring that is substituted at the 2-position with a chlorine atom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Triazines |
|---|
|
Direct Parent |
Chloro-s-triazines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Chloro-s-triazine
- Aryl halide
- Aryl chloride
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
173 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 173 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.0347 mg/mL at 26 °C | Not Available | | LogP | 2.61 | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- atrazine degradation I (aerobic)P141-PWY
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|