|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120228 |
|---|
|
Identification |
|---|
| Name: |
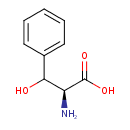
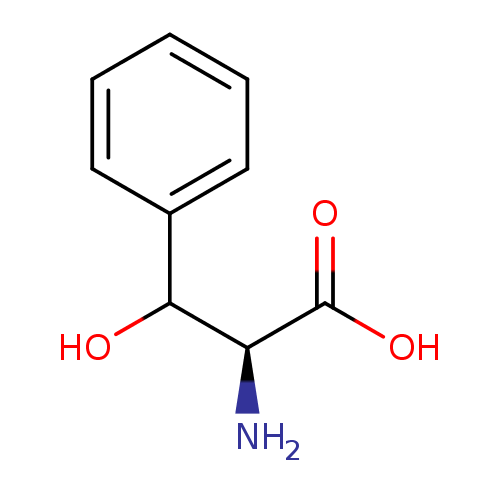
L-threo-3-phenylserine |
|---|
| Description: | Zwitterionic form of L-threo-3-phenylserine arising from transfer of a proton from the carboxy to the α-amino group; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (2S,3S)-2-ammonio-3-hydroxy-3-phenylpropanoate
- L-threo-3-phenylserine
|
|---|
|
Chemical Formula: |
C9H11NO3 |
|---|
| Average Molecular Weight: |
181.191 |
|---|
| Monoisotopic Molecular
Weight: |
182.08171 |
|---|
| InChI Key: |
VHVGNTVUSQUXPS-YUMQZZPRSA-N |
|---|
| InChI: | InChI=1S/C9H11NO3/c10-7(9(12)13)8(11)6-4-2-1-3-5-6/h1-5,7-8,11H,10H2,(H,12,13)/t7-,8-/m0/s1 |
|---|
| CAS
number: |
6254-48-4 |
|---|
| IUPAC Name: | (2S,3S)-2-azaniumyl-3-hydroxy-3-phenylpropanoate |
|---|
|
Traditional IUPAC Name: |
3-phenyl-L-serine |
|---|
| SMILES: | C([O-])(=O)C(C(C1(C=CC=CC=1))O)[N+] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as phenylalanine and derivatives. These are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Phenylalanine and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Phenylalanine or derivatives
- 3-phenylpropanoic-acid
- Alpha-amino acid
- L-alpha-amino acid
- Beta-hydroxy acid
- Aralkylamine
- Monocyclic benzene moiety
- Benzenoid
- Hydroxy acid
- Amino acid
- Secondary alcohol
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organopnictogen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Primary amine
- Primary aliphatic amine
- Aromatic alcohol
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxygen compound
- Amine
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- BRUNS FH, FIEDLER L: Enzymatic cleavage and synthesis of L-threo-beta-phenylserine and L-erythro-beta-Phenyldrine. Nature. 1958 May 31;181(4622):1533-4. [13566053 ]
|
|---|
| Synthesis Reference: |
Bruns, Friedrich H.; Fiedler, Liselore. Enzymic cleavage and synthesis of L-threo-b-phenylserine and L-erythro-b-phenylserine. Biochemische Zeitschrift (1958), 330 324-41. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|