|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120215 |
|---|
|
Identification |
|---|
| Name: |
5-amino-4-imidazolecarboxyamide |
|---|
| Description: | An aminoimidazole in which the amino group is at C-5 with a carboxamido group at C-4. |
|---|
|
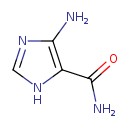
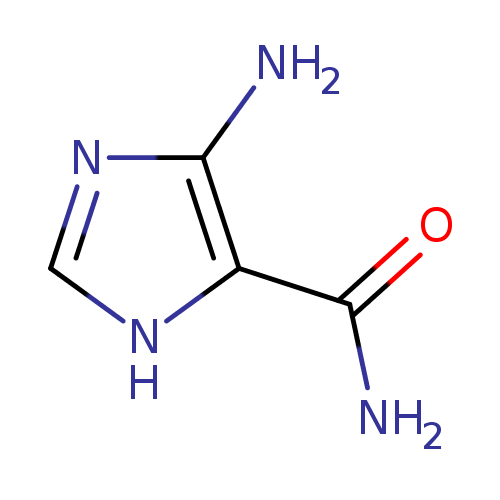
Structure |
|
|---|
| Synonyms: | - 4-amino-5-carbamoylimidazole
- 4-amino-5-imidazolecarboxamide
- 5(4)-amino-4(5)-imidazolecarboxamide
- 5-amino-4-imidazolecarboxyamide
- 5-amino-4-imidazolecarboxyamide
- 5-Amino-4-imidazolecarboxyamide
- 5-aminoimidazole-4-carboxamide
|
|---|
|
Chemical Formula: |
C4H6N4O |
|---|
| Average Molecular Weight: |
126.118 |
|---|
| Monoisotopic Molecular
Weight: |
126.05416 |
|---|
| InChI Key: |
PYNDTGFTPOZGRW-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C4H6N4O/c5-3-2(4(6)9)7-1-8-3/h1-2H,(H2,6,9)(H2,5,7,8) |
|---|
| CAS
number: |
360-97-4 |
|---|
| IUPAC Name: | 5-amino-1H-imidazole-4-carboxamide |
|---|
|
Traditional IUPAC Name: |
4-aminoimidazole-5-carboxamide |
|---|
| SMILES: | C1(=NC(C(N)=O)C(N)=N1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as aminoimidazoles. These are organic compounds containing an amino group linked to an imidazole ring. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Azoles |
|---|
|
Direct Parent |
Aminoimidazoles |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Primary aromatic amine
- Aminoimidazole
- Heteroaromatic compound
- Azacycle
- Carboximidic acid derivative
- Carboximidic acid
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | -1.10 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- STEWART RC, SEVAG MG (1952)4-Amino-5-imidazolecarboxamide; role of carbohydrates as critical factors for its accumulation. Archives of biochemistry and biophysics 41, Pubmed: 12997251
- Choi JH, Ohnishi T, Yamakawa Y, Takeda S, Sekiguchi S, Maruyama W, Yamashita K, Suzuki T, Morita A, Ikka T, Motohashi R, Kiriiwa Y, Tobina H, Asai T, Tokuyama S, Hirai H, Yasuda N, Noguchi K, Asakawa T, Sugiyama S, Kan T, Kawagishi H (2014)The source of "fairy rings": 2-azahypoxanthine and its metabolite found in a novel purine metabolic pathway in plants. Angewandte Chemie (International ed. in English) 53, Pubmed: 24402866
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|