|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120208 |
|---|
|
Identification |
|---|
| Name: |
dimethylglycine |
|---|
| Description: | An amino acid zwitterion resulting from transfer of a proton from the carboxy to the amino group of N,N-dimethylglycine; major species at pH 7.3. |
|---|
|
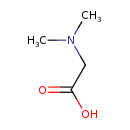
Structure |
|
|---|
| Synonyms: | - (dimethylammonio)acetate
- N,N-dimethylglycine
|
|---|
|
Chemical Formula: |
C4H9NO2 |
|---|
| Average Molecular Weight: |
103.121 |
|---|
| Monoisotopic Molecular
Weight: |
104.07115 |
|---|
| InChI Key: |
FFDGPVCHZBVARC-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C4H9NO2/c1-5(2)3-4(6)7/h3H2,1-2H3,(H,6,7) |
|---|
| CAS
number: |
1118-68-9 |
|---|
| IUPAC Name: | (dimethylazaniumyl)acetate |
|---|
|
Traditional IUPAC Name: |
dimethylglycine |
|---|
| SMILES: | C[N+](CC([O-])=O)C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
Alpha amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-amino acid
- Tertiary amine
- Tertiary aliphatic amine
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Carbonyl group
- Amine
- Hydrocarbon derivative
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
185.5 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 185.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | -2.91 | TSAI,RS ET AL. (1991) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-03gi-3900000000-fe42012cdac3329e6581 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0a4i-9200000000-c3fc59f00bbe1e009053 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0a4i-9000000000-7f6039bf537452ff6c93 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00dj-9100000000-b9c7546951d9f51f58d0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-0udi-2900000000-39eb87f9d7bffb116792 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-0a4r-9100000000-c67140c27d7ca787f694 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-0a4i-9000000000-9c9075d302e11b82ee64 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-0a4i-9000000000-5c3302b240b5bdfde85d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-052f-9000000000-4ec4602699db0863421f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0zfr-6900000000-61f31e5540076abc389d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive | splash10-0a4i-9100000000-635be273cdf7a4cdfe53 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0zfr-5900000000-5ca66452e93663c4d82a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive | splash10-0a4i-9200000000-0e2fb3ac0a1721a846b1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-0udi-0900000000-02624b93137883b214ad | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Binzak BA, Vockley JG, Jenkins RB, Vockley J: Structure and analysis of the human dimethylglycine dehydrogenase gene. Mol Genet Metab. 2000 Mar;69(3):181-7. [10767172 ]
- Binzak BA, Wevers RA, Moolenaar SH, Lee YM, Hwu WL, Poggi-Bach J, Engelke UF, Hoard HM, Vockley JG, Vockley J: Cloning of dimethylglycine dehydrogenase and a new human inborn error of metabolism, dimethylglycine dehydrogenase deficiency. Am J Hum Genet. 2001 Apr;68(4):839-47. Epub 2001 Feb 28. [11231903 ]
- Moolenaar SH, Poggi-Bach J, Engelke UF, Corstiaensen JM, Heerschap A, de Jong JG, Binzak BA, Vockley J, Wevers RA: Defect in dimethylglycine dehydrogenase, a new inborn error of metabolism: NMR spectroscopy study. Clin Chem. 1999 Apr;45(4):459-64. [10102904 ]
- Laryea MD, Steinhagen F, Pawliczek S, Wendel U: Simple method for the routine determination of betaine and N,N-dimethylglycine in blood and urine. Clin Chem. 1998 Sep;44(9):1937-41. [9732980 ]
- McGregor DO, Dellow WJ, Lever M, George PM, Robson RA, Chambers ST: Dimethylglycine accumulates in uremia and predicts elevated plasma homocysteine concentrations. Kidney Int. 2001 Jun;59(6):2267-72. [11380830 ]
- Look MP, Riezler R, Reichel C, Brensing KA, Rockstroh JK, Stabler SP, Spengler U, Berthold HK, Sauerbruch T: Is the increase in serum cystathionine levels in patients with liver cirrhosis a consequence of impaired homocysteine transsulfuration at the level of gamma-cystathionase? Scand J Gastroenterol. 2000 Aug;35(8):866-72. [10994627 ]
- Cicek G, Vuorinen T, Stahle I, Stepanek P, Freudenberg N, Brandsch R: Coxsackievirus B3 infection induces anti-flavoprotein antibodies in mice. Clin Exp Immunol. 2000 Dec;122(3):404-9. [11122247 ]
- Lever M, George PM, Dellow WJ, Scott RS, Chambers ST: Homocysteine, glycine betaine, and N,N-dimethylglycine in patients attending a lipid clinic. Metabolism. 2005 Jan;54(1):1-14. [15562374 ]
- Xia JH, Yu KP, Liu CY, Pan Q, Zheng D, Dai HP: [Molecular clonging of the human dimethyglycine dehydrogenase-like gene (DMGDHL1) from the sarcosinemia critical region at 9q34] Yi Chuan Xue Bao. 1999;26(6):591-7. [10876657 ]
- Jansen M, Hansen TA: Non-growth-associated demethylation of dimethylsulfoniopropionate by (homo)acetogenic bacteria. Appl Environ Microbiol. 2001 Jan;67(1):300-6. [11133459 ]
|
|---|
| Synthesis Reference: |
Lai, Mei-Chin; Wang, Chia-Chi; Chuang, Ming-Jen; Wu, Yen-Chi; Lee, Yu-Chien. Effects of substrate and potassium on the betaine-synthesizing enzyme glycine sarcosine dimethylglycine N-methyltransferase from a halophilic methanoarchaeon Methanohalophilus po |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|