|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120199 |
|---|
|
Identification |
|---|
| Name: |
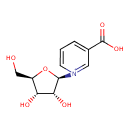
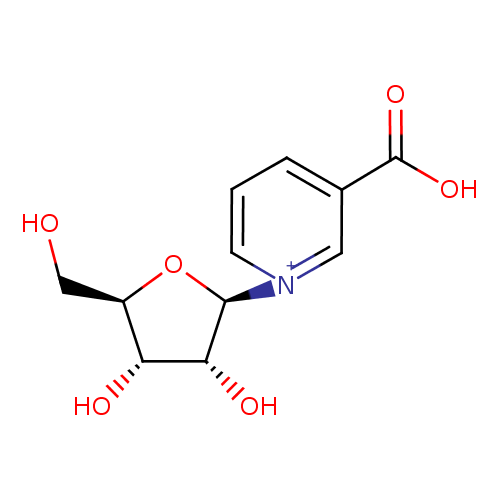
β-D-ribosylnicotinate |
|---|
| Description: | Conjugate base of D-ribosylnicotinic acid. |
|---|
|
Structure |
|
|---|
| Synonyms: | - nicotinic acid riboside
- ribosylnicotinatenicotinic acid
- ribosenicotinate riboside
|
|---|
|
Chemical Formula: |
C11H13NO6 |
|---|
| Average Molecular Weight: |
255.227 |
|---|
| Monoisotopic Molecular
Weight: |
256.08212 |
|---|
| InChI Key: |
PUEDDPCUCPRQNY-ZYUZMQFOSA-N |
|---|
| InChI: | InChI=1S/C11H13NO6/c13-5-7-8(14)9(15)10(18-7)12-3-1-2-6(4-12)11(16)17/h1-4,7-10,13-15H,5H2/t7-,8-,9-,10-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 1-(β-D-ribofuranosyl)pyridinium-3-carboxylate |
|---|
|
Traditional IUPAC Name: |
nicotinic acid riboside |
|---|
| SMILES: | C(O)C1(C(O)C(O)C(O1)[N+]2(C=CC=C(C(=O)[O-])C=2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as glycosylamines. These are compounds consisting of an amine with a beta-N-glycosidic bond to a carbohydrate, thus forming a cyclic hemiaminal ether bond (alpha-amino ether). |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Glycosylamines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- N-glycosyl compound
- Pentose monosaccharide
- Pyridine carboxylic acid
- Pyridine carboxylic acid or derivatives
- Monosaccharide
- Pyridine
- Pyridinium
- Vinylogous amide
- Heteroaromatic compound
- Oxolane
- Secondary alcohol
- Organoheterocyclic compound
- Carboxylic acid derivative
- Oxacycle
- Azacycle
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organonitrogen compound
- Alcohol
- Primary alcohol
- Organic nitrogen compound
- Organic cation
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Nicotinate and Nicotinamide Metabolism pae00760
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|