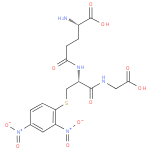
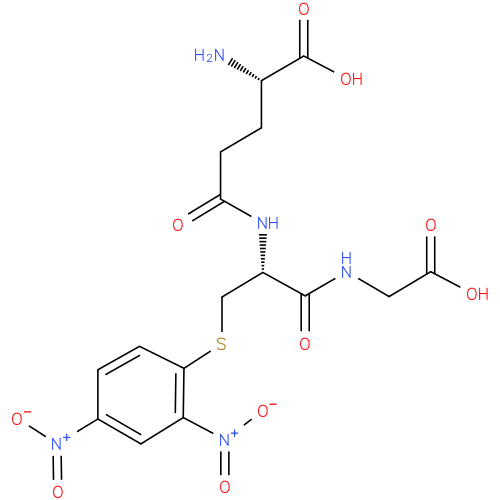
| InChI: | InChI=1S/C16H19N5O10S/c17-9(16(26)27)2-4-13(22)19-10(15(25)18-6-14(23)24)7-32-12-3-1-8(20(28)29)5-11(12)21(30)31/h1,3,5,9-10H,2,4,6-7,17H2,(H,18,25)(H,19,22)(H,23,24)(H,26,27)/p-1/t9-,10-/m0/s1 |
|---|
| References: |
- Awasthi S, Ahmad F, Sharma R, Ahmad H (1992)Reversed-phase chromatographic method for specific determination of glutathione in cultured malignant cells. Journal of chromatography 584, Pubmed: 1484101
- Arias A, Villanueva SS, Ruiz ML, Luquita MG, Veggi LM, Pellegrino JM, Vore M, Catania VA, Mottino AD (2009)Regulation of expression and activity of rat intestinal multidrug resistance-associated protein 2 by cholestatic estrogens. Drug metabolism and disposition: the biological fate of chemicals 37, Pubmed: 19299525
- Diah SK, Smitherman PK, Townsend AJ, Morrow CS (1999)Detoxification of 1-chloro-2,4-dinitrobenzene in MCF7 breast cancer cells expressing glutathione S-transferase P1-1 and/or multidrug resistance protein 1. Toxicology and applied pharmacology 157, Pubmed: 10366541
- Villanueva SS, Perdomo VG, Ruiz ML, Rigalli JP, Arias A, Luquita MG, Vore M, Catania VA, Mottino AD (2012)Effect of glucagon-like peptide 2 on hepatic, renal, and intestinal disposition of 1-chloro-2,4-dinitrobenzene. Drug metabolism and disposition: the biological fate of chemicals 40, Pubmed: 22453052
- Wawrzycka D, Sobczak I, Bartosz G, Bocer T, Ulaszewski S, Goffeau A (2010)Vmr 1p is a novel vacuolar multidrug resistance ABC transporter in Saccharomyces cerevisiae. FEMS yeast research 10, Pubmed: 20846144
- Villanueva SS, Arias A, Ruiz ML, Rigalli JP, Pellegrino JM, Vore M, Catania VA, Mottino AD (2010)Induction of intestinal multidrug resistance-associated protein 2 by glucagon-like Peptide 2 in the rat. The Journal of pharmacology and experimental therapeutics 335, Pubmed: 20719938
- Rigalli JP, Perdomo VG, Luquita MG, Villanueva SS, Arias A, Theile D, Weiss J, Mottino AD, Ruiz ML, Catania VA (2012)Regulation of biotransformation systems and ABC transporters by benznidazole in HepG2 cells: involvement of pregnane X-receptor. PLoS neglected tropical diseases 6, Pubmed: 23272261
- Jin S, Wang XT, Liu L, Yao D, Liu C, Zhang M, Guo HF, Liu XD (2013)P-glycoprotein and multidrug resistance-associated protein 2 are oppositely altered in brain of rats with thioacetamide-induced acute liver failure. Liver international : official journal of the International Association for the Study of the Liver 33, Pubmed: 22925079
- Coleman JW, Yeung JH, Tingle MD, Park BK (1986)Enzyme-linked immunosorbent assay (ELISA) for detection of antibodies to protein-reactive drugs and metabolites: criteria for identification of antibody activity. Detection and hapten specificity of anti-DNP, anti-captopril and anti-sulphanilamidobenzoic acid. Journal of immunological methods 88, Pubmed: 2420897
- Pulaski L, Bartosz G (1995)Effect of inhibitors on the transport of dinitrophenyl-S-glutathione in human erythrocytes. Biochemistry and molecular biology international 36, Pubmed: 7581009
|
|---|