|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120171 |
|---|
|
Identification |
|---|
| Name: |
cobalt-factor III |
|---|
| Description: | A precorrin carboxylic acid anion obtained by deprotonation of the carboxy groups of cobalt(II)-factor III; major species at pH 7.3. |
|---|
|
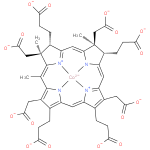
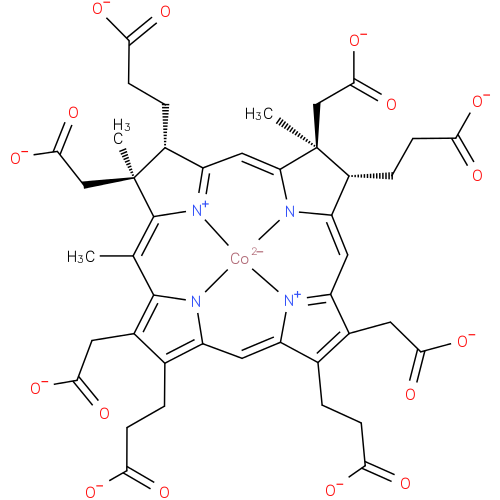
Structure |
|
|---|
| Synonyms: | Not Available |
|---|
|
Chemical Formula: |
C43H38N4O16CO |
|---|
| Average Molecular Weight: |
925.72 |
|---|
| Monoisotopic Molecular
Weight: |
933.22406 |
|---|
| InChI Key: |
SYPADIJAMFFFME-CYGMIEPJSA-D |
|---|
| InChI: | InChI=1S/C43H48N4O16.Co/c1-19-40-23(13-37(58)59)21(5-9-33(50)51)27(46-40)14-26-20(4-8-32(48)49)22(12-36(56)57)28(44-26)15-29-24(6-10-34(52)53)42(2,17-38(60)61)31(45-29)16-30-25(7-11-35(54)55)43(3,18-39(62)63)41(19)47-30;/h14-16,24-25H,4-13,17-18H2,1-3H3,(H10,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63);/q;+4/p-10/t24-,25-,42+,43+;/m1./s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | {3,3',3'',3'''- [(7S,8S,12S,13S)- [(7S,8S,12S,13S)- 3,8,13,17- 3,8,13,17- tetrakis(carboxymethyl)- tetrakis(carboxymethyl)- 8,13,15- 8,13,15- trimethyl- trimethyl- 7,8,12,13- 7,8,12,13- tetrahydroporphyrin- tetrahydroporphyrin- 2,7,12,18- 2,7,12,18- tetrayl- tetrayl- κ4N21,N22,N23,N24]tetrapropanoato(10−)}cobaltate(8−) κ4N21,N22,N23,N24]tetrapropanoato(10−)}cobaltate(8−) |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC1(=C8([N+]4(=C(C=C7(N3(C(=CC5(C(CC(=O)[O-])=C(CCC(=O)[O-])C6(=CC2(=C(CCC(=O)[O-])C(CC(=O)[O-])=C1N2[Co]34[N+]=56))))C(CCC(=O)[O-])C(C)(CC(=O)[O-])7)))C(CCC(=O)[O-])C(C)(CC(=O)[O-])8))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as metallotetrapyrroles. These are polycyclic compounds containing a tetrapyrrole skeleton combined with a metal atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Tetrapyrroles and derivatives |
|---|
| Sub Class | Metallotetrapyrroles |
|---|
|
Direct Parent |
Metallotetrapyrroles |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Metallotetrapyrrole skeleton
- Substituted pyrrole
- Pyrrole
- Pyrrolidine
- Heteroaromatic compound
- Pyrroline
- Carboxylic acid salt
- Carboxylic acid derivative
- Carboxylic acid
- Metalloheterocycle
- Azacycle
- Organic transition metal salt
- Organic metal salt
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic salt
- Carbonyl group
- Organic oxygen compound
- Organic anion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -8 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
| Property | Value | Source |
|---|
| Molecular Weight | 925.722 g/mol | PubChem | | Hydrogen Bond Donor Count | 0 | PubChem | | Hydrogen Bond Acceptor Count | 20 | PubChem | | Rotatable Bond Count | 12 | PubChem | | Exact Mass | 925.161 g/mol | PubChem | | Monoisotopic Mass | 925.161 g/mol | PubChem | | Topological Polar Surface Area | 348 A^2 | PubChem | | Heavy Atom Count | 64 | PubChem | | Formal Charge | -8 | PubChem | | Complexity | 2190 | PubChem | | Isotope Atom Count | 0 | PubChem | | Defined Atom Stereocenter Count | 2 | PubChem | | Undefined Atom Stereocenter Count | 2 | PubChem | | Defined Bond Stereocenter Count | 0 | PubChem | | Undefined Bond Stereocenter Count | 0 | PubChem | | Covalently-Bonded Unit Count | 2 | PubChem |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- cob(II)yrinate a,c-diamide biosynthesis I (early cobalt insertion)PWY-7377
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Moore SJ, Biedendieck R, Lawrence AD, Deery E, Howard MJ, Rigby SE, Warren MJ (2013)Characterization of the enzyme CbiH60 involved in anaerobic ring contraction of the cobalamin (vitamin B12) biosynthetic pathway. The Journal of biological chemistry 288, Pubmed: 23155054
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|