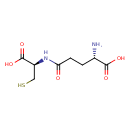
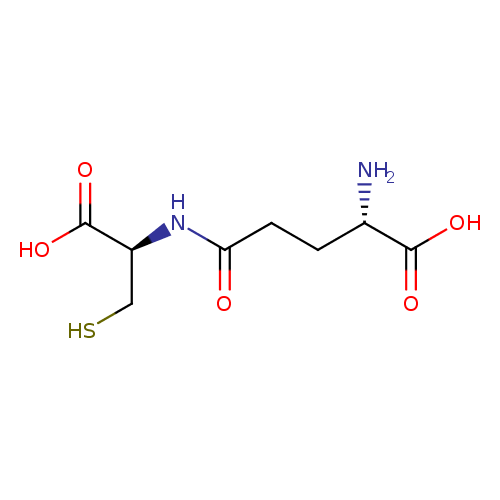
| References: |
- Efferth T, Volm M: Glutathione-related enzymes contribute to resistance of tumor cells and low toxicity in normal organs to artesunate. In Vivo. 2005 Jan-Feb;19(1):225-32. [15796179 ]
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- Andersson A, Isaksson A, Brattstrom L, Hultberg B: Homocysteine and other thiols determined in plasma by HPLC and thiol-specific postcolumn derivatization. Clin Chem. 1993 Aug;39(8):1590-7. [8353942 ]
- Iida M, Yasuhara T, Mochizuki H, Takakura H, Yanagisawa T, Kubo H: Two Japanese brothers with hereditary gamma-glutamyl transpeptidase deficiency. J Inherit Metab Dis. 2005;28(1):49-55. [15702405 ]
- Lochman P, Adam T, Friedecky D, Hlidkova E, Skopkova Z: High-throughput capillary electrophoretic method for determination of total aminothiols in plasma and urine. Electrophoresis. 2003 Apr;24(7-8):1200-7. [12707912 ]
- Atamna H, Ginsburg H: The malaria parasite supplies glutathione to its host cell--investigation of glutathione transport and metabolism in human erythrocytes infected with Plasmodium falciparum. Eur J Biochem. 1997 Dec 15;250(3):670-9. [9461289 ]
- Martensson J: Method for determination of free and total glutathione and gamma-glutamylcysteine concentrations in human leukocytes and plasma. J Chromatogr. 1987 Sep 4;420(1):152-7. [3667817 ]
- Andersson A, Isaksson A, Hultberg B: Homocysteine export from erythrocytes and its implication for plasma sampling. Clin Chem. 1992 Jul;38(7):1311-5. [1623596 ]
- Kaarteenaho-Wiik R, Kinnula VL: Distribution of antioxidant enzymes in developing human lung, respiratory distress syndrome, and bronchopulmonary dysplasia. J Histochem Cytochem. 2004 Sep;52(9):1231-40. [15314090 ]
- Diaz-Hernandez JI, Almeida A, Delgado-Esteban M, Fernandez E, Bolanos JP: Knockdown of glutamate-cysteine ligase by small hairpin RNA reveals that both catalytic and modulatory subunits are essential for the survival of primary neurons. J Biol Chem. 2005 Nov 25;280(47):38992-9001. Epub 2005 Sep 23. [16183645 ]
- Levonen AL, Lapatto R, Saksela M, Raivio KO: Expression of gamma-glutamylcysteine synthetase during development. Pediatr Res. 2000 Feb;47(2):266-70. [10674357 ]
- Martensson J, Kagedal B, Larsson A: Sulphur amino-acid degradation in subjects with hereditary glutathione synthetase deficiency (5-oxoprolinuria). Eur J Clin Invest. 1985 Dec;15(6):371-4. [3938407 ]
- Zinellu A, Sotgia S, Posadino AM, Pasciu V, Perino MG, Tadolini B, Deiana L, Carru C: Highly sensitive simultaneous detection of cultured cellular thiols by laser induced fluorescence-capillary electrophoresis. Electrophoresis. 2005 Mar;26(6):1063-70. [15706569 ]
- Njalsson R, Ristoff E, Carlsson K, Winkler A, Larsson A, Norgren S: Genotype, enzyme activity, glutathione level, and clinical phenotype in patients with glutathione synthetase deficiency. Hum Genet. 2005 Apr;116(5):384-9. Epub 2005 Feb 17. [15717202 ]
- Lou MF, Dickerson JE Jr, Tung WH, Wolfe JK, Chylack LT Jr: Correlation of nuclear color and opalescence with protein S-thiolation in human lenses. Exp Eye Res. 1999 May;68(5):547-52. [10328968 ]
|
|---|