|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120151 |
|---|
|
Identification |
|---|
| Name: |
coumarinate |
|---|
| Description: | A 2-coumarate that is the conjugate base of cis-2-coumaric acid. |
|---|
|
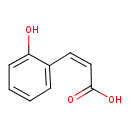
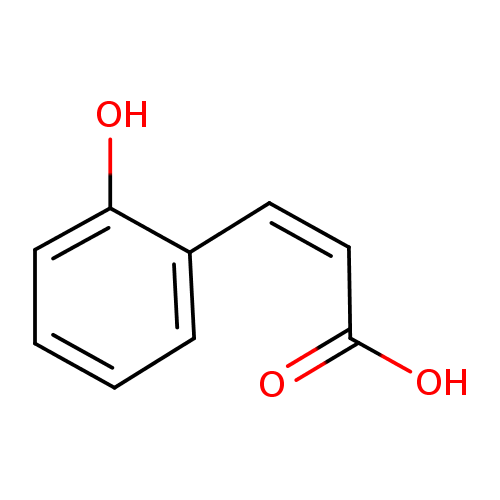
Structure |
|
|---|
| Synonyms: | - (2Z)-3-(2-hydroxyphenyl)acrylate
- cis-2-coumarate
- cis-2-hydroxycinnamate
|
|---|
|
Chemical Formula: |
C9H7O3 |
|---|
| Average Molecular Weight: |
163.152 |
|---|
| Monoisotopic Molecular
Weight: |
164.04735 |
|---|
| InChI Key: |
PMOWTIHVNWZYFI-WAYWQWQTSA-M |
|---|
| InChI: | InChI=1S/C9H8O3/c10-8-4-2-1-3-7(8)5-6-9(11)12/h1-6,10H,(H,11,12)/p-1/b6-5- |
|---|
| CAS
number: |
495-79-4 |
|---|
| IUPAC Name: | (2Z)-3-(2-hydroxyphenyl)prop-2-enoate |
|---|
|
Traditional IUPAC Name: |
2-coumarinate |
|---|
| SMILES: | C(C=CC1(=C(C=CC=C1)O))(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as hydroxycinnamic acids. These are compounds containing an cinnamic acid where the benzene ring is hydroxylated. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
|
Class |
Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
|
Direct Parent |
Hydroxycinnamic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydroxycinnamic acid
- Coumaric acid or derivatives
- Coumaric acid
- Cinnamic acid
- Phenylpropene
- Styrene
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|