|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120149 |
|---|
|
Identification |
|---|
| Name: |
phenylglyoxylate |
|---|
| Description: | A member of the class of glyoxylates, that is obtained by removal of a proton from the carboxylic acid group of phenylglyoxylic acid. |
|---|
|
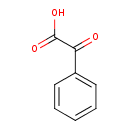
Structure |
|
|---|
| Synonyms: | - benzoylformic acid anion
- phenylglyoxylate
- phenylglyoxylic acid anion
|
|---|
|
Chemical Formula: |
C8H5O3 |
|---|
| Average Molecular Weight: |
149.126 |
|---|
| Monoisotopic Molecular
Weight: |
150.0317 |
|---|
| InChI Key: |
FAQJJMHZNSSFSM-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C8H6O3/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5H,(H,10,11)/p-1 |
|---|
| CAS
number: |
611-73-4 |
|---|
| IUPAC Name: | oxo(phenyl)acetate |
|---|
|
Traditional IUPAC Name: |
benzoylformic acid |
|---|
| SMILES: | C(=O)(C(C1(C=CC=CC=1))=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as benzoyl derivatives. These are organic compounds containing an acyl moiety of benzoic acid with the formula (C6H5CO-). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
|
Class |
Benzene and substituted derivatives |
|---|
| Sub Class | Benzoyl derivatives |
|---|
|
Direct Parent |
Benzoyl derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Aryl ketone
- Benzoyl
- Keto acid
- Alpha-keto acid
- Alpha-hydroxy ketone
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
66 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 66 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 920.0 mg/mL at 0 °C | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0zfr-1900000000-15499c77fbdf6aba2c6f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-004i-9300000000-c84b000626073ef1eb7e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-004i-9100000000-0d840af6ff6db1d61038 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0002-0900000000-22fd9c3f15b7011e1779 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-004i-9000000000-eb5833f7b7cb1312e0fb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-004i-9000000000-518533199e7a59cb1b17 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-004i-9000000000-a7f64053378dba21530c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-004i-9000000000-77eed9b73d7a3aa5b334 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Lacroix C, Inger F, Menager S, Lafont O: [Simultaneous determination of urinary hippuric acid and o-, m-, p-methylhippuric acids with liquid chromatography]. J Chromatogr. 1986 Oct 31;382:275-9. [3782394 ]
- Sakai T, Takeuchi Y, Ikeya Y, Araki T, Ushio K: [Method for simultaneous determination of six metabolites of toluene, xylene and ethylbenzene, and its application to exposure monitoring of workers in a printing factory with gravure machines]. Sangyo Igaku. 1989 Jan;31(1):9-16. [2739101 ]
|
|---|
| Synthesis Reference: |
Hurd, Charles D.; McNamee, R. W.; Green, Frank O. Benzoylformic acid from styrene. Journal of the American Chemical Society (1939), 61 2979-80. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|