|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120148 |
|---|
|
Identification |
|---|
| Name: |
4-hydroxy-2-nonenal |
|---|
| Description: | An enal consisting of non-2-ene having an oxo group at the 1-position and a hydroxy group at the 4-position. |
|---|
|
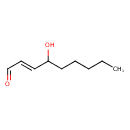
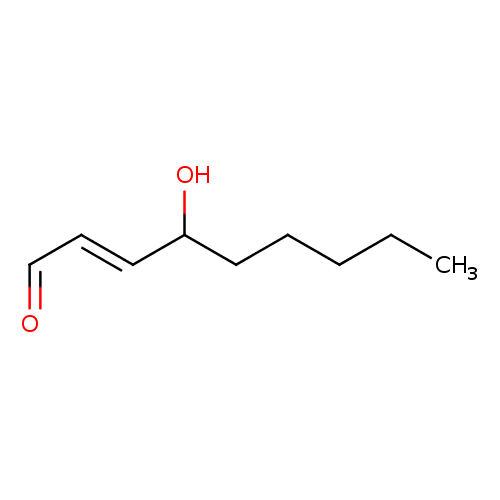
Structure |
|
|---|
| Synonyms: | - 4-HNE
- 4-Hydroxy-2,3-nonenal
- 4-Hydroxy-2-nonenal
- 4-Hydroxynonenal
- HNE
|
|---|
|
Chemical Formula: |
C9H16O2 |
|---|
| Average Molecular Weight: |
156.224 |
|---|
| Monoisotopic Molecular
Weight: |
156.11504 |
|---|
| InChI Key: |
JVJFIQYAHPMBBX-FNORWQNLSA-N |
|---|
| InChI: | InChI=1S/C9H16O2/c1-2-3-4-6-9(11)7-5-8-10/h5,7-9,11H,2-4,6H2,1H3/b7-5+ |
|---|
| CAS
number: |
75899-68-2 |
|---|
| IUPAC Name: | 4-hydroxynon-2-enal |
|---|
|
Traditional IUPAC Name: |
trans-4-hydroxy-2-nonenal |
|---|
| SMILES: | CCCCCC(O)[CH]=CC=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as fatty alcohols. These are aliphatic alcohols consisting of a chain of a least six carbon atoms. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Lipids and lipid-like molecules |
|---|
| Sub Class | Fatty Acyls |
|---|
|
Direct Parent |
Fatty alcohols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Fatty alcohol
- Medium-chain aldehyde
- Enal
- Alpha,beta-unsaturated aldehyde
- Secondary alcohol
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aldehyde
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, Awasthi S: Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med. 2004 Sep 1;37(5):607-19. [15288119 ]
- Selley ML, Close DR, Stern SE: The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer's disease. Neurobiol Aging. 2002 May-Jun;23(3):383-8. [11959400 ]
- Selley ML: (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson's disease. Free Radic Biol Med. 1998 Jul 15;25(2):169-74. [9667492 ]
|
|---|
| Synthesis Reference: |
Esterbauer, Hermann; Benedetti, Angelo; Lang, Johanna; Fulceri, Rosella; Fauler, Gunther; Comporti, Mario. Studies on the mechanism of formation of 4-hydroxynonenal during microsomal lipid peroxidation. Biochimica et Biophysica Acta, Lipids and Lipid Metabolism (1986), 876(1), 154-66. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|