|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120139 |
|---|
|
Identification |
|---|
| Name: |
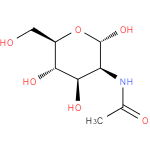
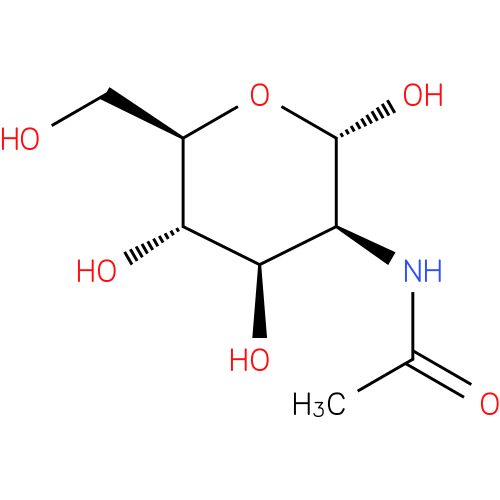
N-acetyl-α-D-mannosamine |
|---|
| Description: | An N-acetylmannosamine having pyranose form and α-D-configuration. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-(ACETYLAMINO)-2-DEOXY-ALPHA-D-MANNOPYRANOSE
- 2-acetamido-2-deoxy-α-D-mannose
- 2-acetylamino-α-D-2-deoxy-mannopyranose
- α-D-ManAc
- α-D-ManpAc
- α-ManAc
|
|---|
|
Chemical Formula: |
C8H15NO6 |
|---|
| Average Molecular Weight: |
221.21 |
|---|
| Monoisotopic Molecular
Weight: |
221.08994 |
|---|
| InChI Key: |
OVRNDRQMDRJTHS-UOLFYFMNSA-N |
|---|
| InChI: | InChI=1S/C8H15NO6/c1-3(11)9-5-7(13)6(12)4(2-10)15-8(5)14/h4-8,10,12-14H,2H2,1H3,(H,9,11)/t4-,5+,6-,7-,8+/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2-acetamido-2-deoxy-α-D-mannopyranose |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC(=O)NC1(C(O)OC(CO)C(O)C(O)1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
|
Class |
Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
|
Direct Parent |
Acylaminosugars |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Acylaminosugar
- N-acyl-alpha-hexosamine
- Hexose monosaccharide
- Monosaccharide
- Oxane
- Acetamide
- Carboxamide group
- Hemiacetal
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Organonitrogen compound
- Primary alcohol
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
- N-acyl-hexosamine (CHEBI:7203)
- an N-acetyl-D-hexosamine (N-ACETYL-D-MANNOSAMINE)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Berkin A, Coxon B, Pozsgay V (2002)Towards a synthetic glycoconjugate vaccine against Neisseria meningitidis A. Chemistry (Weinheim an der Bergstrasse, Germany) 8, Pubmed: 12355530
- Lipkind GM, Shashkov AS, Knirel YA, Vinogradov EV, Kochetkov NK (1988)A computer-assisted structural analysis of regular polysaccharides on the basis of 13C-n.m.r. data. Carbohydrate research 175, Pubmed: 3378242
- Coxon B (2005)Deuterium isotope effects in carbohydrates revisited. Cryoprobe studies of the anomerization and NH to ND deuterium isotope induced 13C NMR chemical shifts of acetamidodeoxy and aminodeoxy sugars. Carbohydrate research 340, Pubmed: 15936003
- Wada M, Hsu CC, Franke D, Mitchell M, Heine A, Wilson I, Wong CH (2003)Directed evolution of N-acetylneuraminic acid aldolase to catalyze enantiomeric aldol reactions. Bioorganic & medicinal chemistry 11, Pubmed: 12670660
- Pan Y, Ayani T, Nadas J, Wen S, Guo Z (2004)Accessibility of N-acyl-D-mannosamines to N-acetyl-D-neuraminic acid aldolase. Carbohydrate research 339, Pubmed: 15280054
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|