|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120138 |
|---|
|
Identification |
|---|
| Name: |
5-fluorouracil |
|---|
| Description: | A nucleobase analogue that is uracil in which the hydrogen at position 5 is replaced by fluorine. It is an antineoplastic agent which acts as an antimetabolite - following conversion to the active deoxynucleotide, it inhibits DNA synthesis (by blocking the conversion of deoxyuridylic acid to thymidylic acid by the cellular enzyme thymidylate synthetase) and so slows tumour growth. |
|---|
|
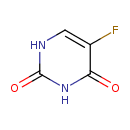
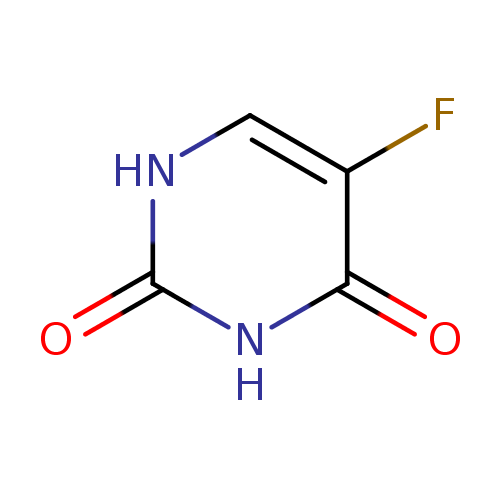
Structure |
|
|---|
| Synonyms: | - 5-Fluoracil
- 5-Fluoropyrimidine-2,4-dione
- 5-Fluorouracil
- 5-FU
- Fluorouracil
|
|---|
|
Chemical Formula: |
C4H3N2O2F |
|---|
| Average Molecular Weight: |
130.078 |
|---|
| Monoisotopic Molecular
Weight: |
130.01785 |
|---|
| InChI Key: |
GHASVSINZRGABV-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C4H3FN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9) |
|---|
| CAS
number: |
51-21-8 |
|---|
| IUPAC Name: | 5-fluoropyrimidine-2,4(1H,3H)-dione |
|---|
|
Traditional IUPAC Name: |
fluorouracil |
|---|
| SMILES: | C1(=C(C(=O)NC(N1)=O)F) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as halopyrimidines. These are aromatic compounds containing a halogen atom linked to a pyrimidine ring. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Diazines |
|---|
|
Direct Parent |
Halopyrimidines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydroxypyrimidine
- Halopyrimidine
- Aryl halide
- Aryl fluoride
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
280 - 282 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 280 - 282 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 5.86e+00 g/L | Not Available | | LogP | -0.8 | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Glavas-Dodov M, Fredro-Kumbaradzi E, Goracinova K, Calis S, Simonoska M, Hincal AA (2003)5-Fluorouracil in topical liposome gels for anticancer treatment--formulation and evaluation. Acta pharmaceutica (Zagreb, Croatia) 53, Pubmed: 14769231
- Lawrence TS, Blackstock AW, McGinn C (2003)The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Seminars in radiation oncology 13, Pubmed: 12520460
- Yoshisue K, Nagayama S, Shindo T, Kawaguchi Y (2001)Effects of 5-fluorouracil on the drug-metabolizing enzymes of the small intestine and the consequent drug interaction with nifedipine in rats. The Journal of pharmacology and experimental therapeutics 297, Pubmed: 11356943
- Yasumatsu R, Nakashima T, Uryu H, Ayada T, Wakasaki T, Kogo R, Masuda M, Fukushima M, Komune S (2009)Correlations between thymidylate synthase expression and chemosensitivity to 5-fluorouracil, cell proliferation and clinical outcome in head and neck squamous cell carcinoma. Chemotherapy 55, Pubmed: 19023200
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|