|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120121 |
|---|
|
Identification |
|---|
| Name: |
chitotriose |
|---|
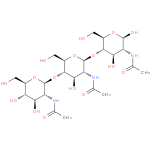
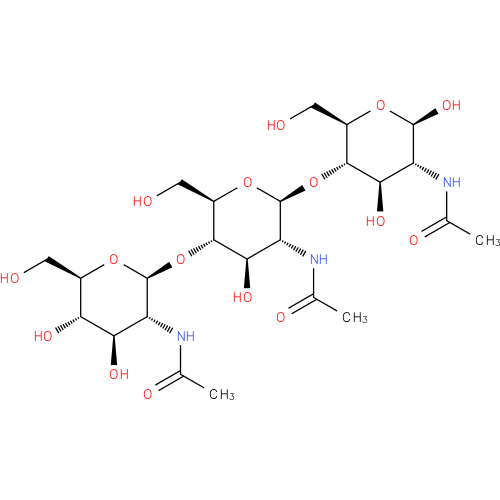
| Description: | A linear amino trisaccharide consisting of three N-acetyl-β-D-glucosamine residues linked (1→4). |
|---|
|
Structure |
|
|---|
| Synonyms: | - (β)-N,N',N''-triacetyl chitotriose
- β-
 D- D- GlcNAc- GlcNAc- (1→4)- (1→4)- β- β- D- D- GlcNAc- GlcNAc- (1→4)- (1→4)- β- β- D- D- GlcNAc GlcNAc - β-N,N',N''-triacetylchitotriose
- GlcNAcb1-4GlcNAcb1-4GlcNAcb
- GlcNAcβ1-4GlcNAcβ1-4GlcNAcβ
- N,N',N''-triacetyl chitotriose β-anomer
- N-
 acetyl- acetyl- β- β- D- D- glucosaminyl- glucosaminyl- (1→4)- (1→4)- N- N- acetyl- acetyl- β- β- D- D- glucosaminyl- glucosaminyl- (1→4)- (1→4)- N- N- acetyl- acetyl- β- β- D- D- glucosamine glucosamine
|
|---|
|
Chemical Formula: |
C24H41N3O16 |
|---|
| Average Molecular Weight: |
627.598 |
|---|
| Monoisotopic Molecular
Weight: |
627.24866 |
|---|
| InChI Key: |
WZZVUHWLNMNWLW-MEWKLCDLSA-N |
|---|
| InChI: | InChI=1S/C24H41N3O16/c1-7(31)25-13-18(36)20(11(5-29)39-22(13)38)42-24-15(27-9(3)33)19(37)21(12(6-30)41-24)43-23-14(26-8(2)32)17(35)16(34)10(4-28)40-23/h10-24,28-30,34-38H,4-6H2,1-3H3,(H,25,31)(H,26,32)(H,27,33)/t10-,11-,12-,13-,14-,15-,16-,17-,18-,19-,20-,21-,22?,23+,24+/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2- acetamido- acetamido- 2- 2- deoxy- deoxy- β- β- D- D- glucopyranosyl- glucopyranosyl- (1→4)- (1→4)- 2- 2- acetamido- acetamido- 2- 2- deoxy- deoxy- β- β- D- D- glucopyranosyl- glucopyranosyl- (1→4)- (1→4)- 2- 2- acetamido- acetamido- 2- 2- deoxy- deoxy- β- β- D- D- glucopyranose glucopyranose |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC(=O)NC1(C(O)OC(CO)C(C(O)1)OC2(C(NC(C)=O)C(O)C(C(CO)O2)OC3(OC(C(O)C(O)C(NC(C)=O)3)CO))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
|
Class |
Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
|
Direct Parent |
Acylaminosugars |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Oligosaccharide
- Acylaminosugar
- N-acyl-alpha-hexosamine
- Glycosyl compound
- O-glycosyl compound
- Oxane
- Acetamide
- Carboxamide group
- Hemiacetal
- Secondary carboxylic acid amide
- Secondary alcohol
- Acetal
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Carbonyl group
- Alcohol
- Primary alcohol
- Organonitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
- a chitodextrin (CPD-13227)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- von Gunten S, Smith DF, Cummings RD, Riedel S, Miescher S, Schaub A, Hamilton RG, Bochner BS (2009)Intravenous immunoglobulin contains a broad repertoire of anticarbohydrate antibodies that is not restricted to the IgG2 subclass. The Journal of allergy and clinical immunology 123, Pubmed: 19443021
- Schneider C, Smith DF, Cummings RD, Boligan KF, Hamilton RG, Bochner BS, Miescher S, Simon HU, Pashov A, Vassilev T, von Gunten S (2015)The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Science translational medicine 7, Pubmed: 25568069
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|