|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120118 |
|---|
|
Identification |
|---|
| Name: |
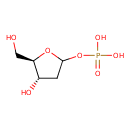
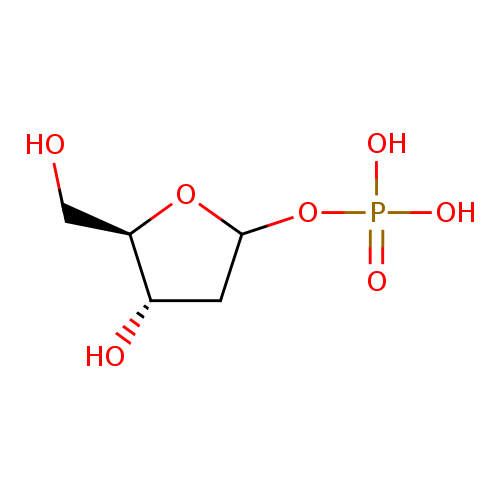
2-deoxy-α-D-ribose 1-phosphate |
|---|
| Description: | Dianion of 2-deoxy-α-D-ribose 1-phosphate. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-deoxy-1-O-phosphonato-α-D-erythro-pentofuranose
- 2-deoxy-α-D-ribose 1-phosphate
|
|---|
|
Chemical Formula: |
C5H9O7P |
|---|
| Average Molecular Weight: |
212.096 |
|---|
| Monoisotopic Molecular
Weight: |
214.02425 |
|---|
| InChI Key: |
KBDKAJNTYKVSEK-VPENINKCSA-L |
|---|
| InChI: | InChI=1S/C5H11O7P/c6-2-4-3(7)1-5(11-4)12-13(8,9)10/h3-7H,1-2H2,(H2,8,9,10)/p-2/t3-,4+,5+/m0/s1 |
|---|
| CAS
number: |
17210-42-3 |
|---|
| IUPAC Name: | 2-deoxy-α-D-erythro-pentofuranose 1-phosphate |
|---|
|
Traditional IUPAC Name: |
deoxyribose-1-phosphate |
|---|
| SMILES: | C1(C(O)C(CO)OC1OP(=O)([O-])[O-]) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Pentoses |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pentose monosaccharide
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Oxolane
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Holmberg I, Stal P, Hamberg M: Quantitative determination of 8-hydroxy-2'-deoxyguanosine in human urine by isotope dilution mass spectrometry: normal levels in hemochromatosis. Free Radic Biol Med. 1999 Jan;26(1-2):129-35. [9890648 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|