|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120116 |
|---|
|
Identification |
|---|
| Name: |
indole-3-acetate |
|---|
| Description: | An indol-3-yl carboxylic acid anion that is the conjugate base of indole-3-acetic acid. |
|---|
|
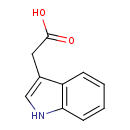
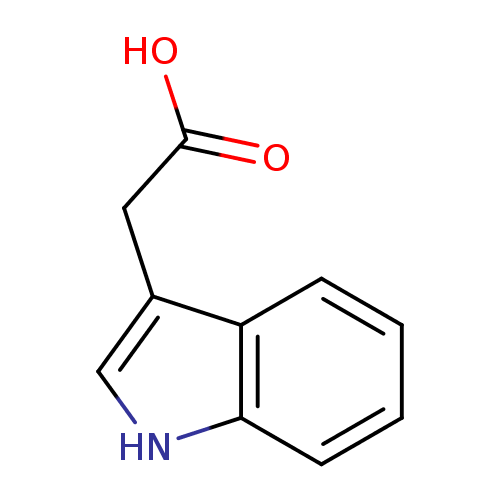
Structure |
|
|---|
| Synonyms: | - (indol-3-yl)acetate
- 2-(indol-3-yl)ethanoate
|
|---|
|
Chemical Formula: |
C10H8NO2 |
|---|
| Average Molecular Weight: |
174.179 |
|---|
| Monoisotopic Molecular
Weight: |
175.06332 |
|---|
| InChI Key: |
SEOVTRFCIGRIMH-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C10H9NO2/c12-10(13)5-7-6-11-9-4-2-1-3-8(7)9/h1-4,6,11H,5H2,(H,12,13)/p-1 |
|---|
| CAS
number: |
87-51-4 |
|---|
| IUPAC Name: | 1H-indol-3-ylacetate |
|---|
|
Traditional IUPAC Name: |
?-indole-3-acetic acid |
|---|
| SMILES: | C([O-])(=O)CC1(=CNC2(C=CC=CC1=2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as indole-3-acetic acid derivatives. These are compounds containing an acetic acid (or a derivative) linked to the C3 carbon atom of an indole. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Indoles and derivatives |
|---|
| Sub Class | Indolyl carboxylic acids and derivatives |
|---|
|
Direct Parent |
Indole-3-acetic acid derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Indole-3-acetic acid derivative
- 3-alkylindole
- Indole
- Substituted pyrrole
- Benzenoid
- Heteroaromatic compound
- Pyrrole
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Carbonyl group
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
168.5 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 168.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 1.5 mg/mL | Not Available | | LogP | 1.41 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-0udi-0391000000-7581f14fe5be5b2b2954 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-0udi-0691000000-de9ac4f748d50db109ea | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-0udi-0591000000-9687f83d1372abe23c3c | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-0udi-1793100000-7c78003038436ec5a902 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (1 TMS) | splash10-00ai-7910000000-4aa7b8244f32048c76bc | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-0fk9-9250000000-a5f931fc3292056dba65 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-001i-1920000000-f0ecee61454a589493af | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-0udi-1692000000-ce863a1ca2a657cb41d5 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-004i-0900000000-f6dbb01a35af3042d126 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-004i-0900000000-2ae231b7d0e2cd50aed8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-004i-6900000000-9eae14faa16b8f8259da | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80) , Positive | splash10-003r-0900000000-1edcb4977a52155bc130 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-00e9-0900000000-187b48f2258823cbc6a2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-001i-0900000000-30b7a73fa446d0e3c8d3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-004i-0900000000-bbe0fb5a48f89ea6e383 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-004i-0900000000-97850f400d80de278334 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-004i-0900000000-600545759ef108827b9e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-0kxr-5900000000-ba2eed29832f9ee48921 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-004l-5900000000-a0b30710f83e53b6f3db | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-001i-9500000000-146ac0a20f9d53e76291 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-053r-9600000000-d9258b3c6b5c6f748f6e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-004i-9300000000-b59628beb41b424daf4a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (UPLC Waters, Quattro Ultima Pt Micromass) , Positive (Annotated) | splash10-004i-0900000000-755373c9248cfb6425fc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-004i-0900000000-2b3df7a1dd85faea6705 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive | splash10-003r-0900000000-9522ab089ab89b64f96a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-001i-0900000000-b735e95cb23091491c2e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-2a3dd24f136523e6ce1e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-053r-0900000000-77436493836245345cb8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00lr-1900000000-4e0b6f24d03c0b25800f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00e9-0900000000-1d045a56f3669a1c9391 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05ai-0900000000-518778c36bacbf77b901 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a5c-3900000000-323fc64084836756be30 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-2a3dd24f136523e6ce1e | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-001i-1900000000-3ffc47eb6c977956ad93 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Guneral F, Bachmann C: Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin Chem. 1994 Jun;40(6):862-6. [8087979 ]
- Igari T, Tsuchizawa M, Shimamura T: Alteration of tryptophan metabolism in the synovial fluid of patients with rheumatoid arthritis and osteoarthritis. Tohoku J Exp Med. 1987 Oct;153(2):79-86. [3500530 ]
- Carpenter LL, Anderson GM, Siniscalchi JM, Chappell PB, Price LH: Acute changes in cerebrospinal fluid 5-HIAA following oral paroxetine challenge in healthy humans. Neuropsychopharmacology. 2003 Feb;28(2):339-47. [12589387 ]
- Owens MJ, Nemeroff CB: Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994 Feb;40(2):288-95. [7508830 ]
- Tu JB, Wong CY: Serotonin metabolism in normal and abnormal infants during the perinatal period. Biol Neonate. 1976;29(3-4):187-93. [133735 ]
- Blennow K, Wallin A, Gottfries CG, Mansson JE, Svennerholm L: Concentration gradients for monoamine metabolites in lumbar cerebrospinal fluid. J Neural Transm Park Dis Dement Sect. 1993;5(1):5-15. [7679905 ]
- Morgan WW, Grant RW: Increased rate of disappearance of serum probenecid in barbital dependent rats. Eur J Pharmacol. 1976 Dec;40(2):349-57. [1033074 ]
- Jellinger K, Riederer P: Brain monoamines in metabolic (endotoxic) coma. A preliminary biochemical study in human postmortem material. J Neural Transm. 1977;41(4):275-86. [925688 ]
- Sarrias MJ, Cabre P, Martinez E, Artigas F: Relationship between serotoninergic measures in blood and cerebrospinal fluid simultaneously obtained in humans. J Neurochem. 1990 Mar;54(3):783-6. [1689378 ]
- Kema IP, Meijer WG, Meiborg G, Ooms B, Willemse PH, de Vries EG: Profiling of tryptophan-related plasma indoles in patients with carcinoid tumors by automated, on-line, solid-phase extraction and HPLC with fluorescence detection. Clin Chem. 2001 Oct;47(10):1811-20. [11568091 ]
- Bai F, Jones DC, Lau SS, Monks TJ: Serotonergic neurotoxicity of 3,4-(+/-)-methylenedioxyamphetamine and 3,4-(+/-)-methylendioxymethamphetamine (ecstasy) is potentiated by inhibition of gamma-glutamyl transpeptidase. Chem Res Toxicol. 2001 Jul;14(7):863-70. [11453733 ]
- Ridges AP, Bishop FM, Lawton K, Goldberg IJ: Amine metabolism, thyroid function and response to clomipramine and maprotiline medication in depression. Postgrad Med J. 1980;56 Suppl 1:37-41. [6156444 ]
- Raghuram TC, Krishnaswamy K: Serotonin metabolism is pellagra. Arch Neurol. 1975 Oct;32(10):708-10. [1180737 ]
- Carling RS, Degg TJ, Allen KR, Bax ND, Barth JH: Evaluation of whole blood serotonin and plasma and urine 5-hydroxyindole acetic acid in diagnosis of carcinoid disease. Ann Clin Biochem. 2002 Nov;39(Pt 6):577-82. [12564839 ]
- Taniguchi K, Okatani Y, Sagara Y: Serotonin metabolism in the fetus in preeclampsia. Asia Oceania J Obstet Gynaecol. 1994 Mar;20(1):77-86. [7513511 ]
- Russo S, Boon JC, Kema IP, Willemse PH, den Boer JA, Korf J, de Vries EG: Patients with carcinoid syndrome exhibit symptoms of aggressive impulse dysregulation. Psychosom Med. 2004 May-Jun;66(3):422-5. [15184706 ]
- Igari T, Shimamura T: Serotonin metabolism and its enzymic activities in joint diseases. Clin Orthop Relat Res. 1979 Mar-Apr;(139):232-49. [455840 ]
- Bearcroft CP, Perrett D, Farthing MJ: Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut. 1998 Jan;42(1):42-6. [9505884 ]
- Ilkhanizadeh B, Owji AA, Tavangar SM, Vasei M, Tabei SM: Spot urine 5-hydroxy indole acetic acid and acute appendicitis. Hepatogastroenterology. 2001 May-Jun;48(39):609-13. [11462886 ]
- Apak S, Kazez A, Ozel SK, Ustundag B, Akpolat N, Kizirgil A: Spot urine 5-hydroxyindoleacetic acid levels in the early diagnosis of acute appendicitis. J Pediatr Surg. 2005 Sep;40(9):1436-9. [16150345 ]
- WEISSBACH H, KING W, SJOERDSMA A, UDENFRIEND S: Formation of indole-3-acetic acid and tryptamine in animals: a method for estimation of indole-3-acetic acid in tissues. J Biol Chem. 1959 Jan;234(1):81-6. [13610897 ]
- Folkes LK, Wardman P: Oxidative activation of indole-3-acetic acids to cytotoxic species- a potential new role for plant auxins in cancer therapy. Biochem Pharmacol. 2001 Jan 15;61(2):129-36. [11163327 ]
|
|---|
| Synthesis Reference: |
Snyder, H. R.; Pilgrim, Frederick J. Preparation of 3-indoleacetic acid; new synthesis of tryptophol. Journal of the American Chemical Society (1948), 70 3770-1. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|