|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120099 |
|---|
|
Identification |
|---|
| Name: |
glutathioselenol |
|---|
| Description: | A glutathione derivative that is glutathione in which the hydrogen attached to the sulfur is replaced by a selenol group. |
|---|
|
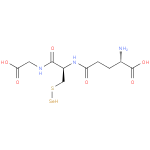
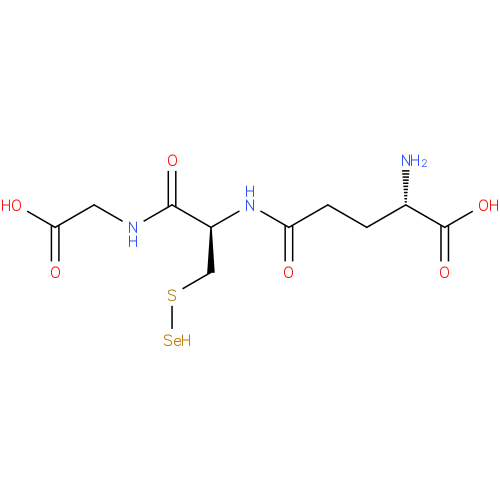
Structure |
|
|---|
| Synonyms: | - GSSeH
- selenoglutathione persulfide
- selenoglutathione
|
|---|
|
Chemical Formula: |
C10H16N3O6SSE |
|---|
| Average Molecular Weight: |
384.265 |
|---|
| Monoisotopic Molecular
Weight: |
388.00815 |
|---|
| InChI Key: |
UUYVRXVWXDDDGX-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C10H17N3O6SSe/c11-5(10(18)19)1-2-7(14)13-6(4-20-21)9(17)12-3-8(15)16/h5-6,21H,1-4,11H2,(H,12,17)(H,13,14)(H,15,16)(H,18,19)/p-1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | L-γ-glutamyl-S-selanyl-L-cysteinylglycine |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | C(S[Se])C(NC(=O)CCC([N+])C(=O)[O-])C(=O)NCC(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
Oligopeptides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-oligopeptide
- Gamma-glutamyl alpha peptide
- Glutamine or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Alpha-amino acid amide
- Cysteine or derivatives
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- N-acyl-amine
- Fatty acyl
- Fatty amide
- Fatty acid
- Dicarboxylic acid or derivatives
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid salt
- Carboxylic acid
- Sulfenyl compound
- Organosulfur compound
- Organic nitrogen compound
- Organic salt
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
Not Available |
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Turner RJ, Weiner JH, Taylor DE (1998)Selenium metabolism in Escherichia coli. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 11, Pubmed: 9850565
- Hsieh HS, Ganther HE (1975)Acid-volatile selenium formation catalyzed by glutathione reductase. Biochemistry 14, Pubmed: 235962
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|