|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120078 |
|---|
|
Identification |
|---|
| Name: |
uridine 3'-monophosphate |
|---|
| Description: | A nucleoside 3'-phosphate(2−) obtained by deprotonation of the phosphate OH groups of uridine 3'-monophosphate (UMP) It is the predominant species at physiological pH. |
|---|
|
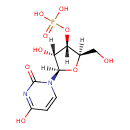
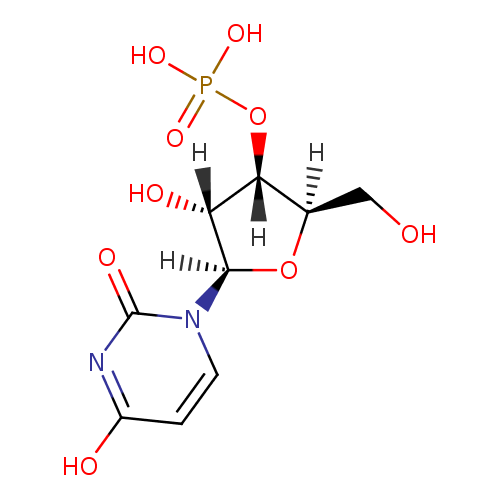
Structure |
|
|---|
| Synonyms: | - 3'-UMP
- 3'-uridylate
- uridine 3'-monophosphate
|
|---|
|
Chemical Formula: |
C9H11N2O9P |
|---|
| Average Molecular Weight: |
322.168 |
|---|
| Monoisotopic Molecular
Weight: |
324.03586 |
|---|
| InChI Key: |
FOGRQMPFHUHIGU-XVFCMESISA-L |
|---|
| InChI: | InChI=1S/C9H13N2O9P/c12-3-4-7(20-21(16,17)18)6(14)8(19-4)11-2-1-5(13)10-9(11)15/h1-2,4,6-8,12,14H,3H2,(H,10,13,15)(H2,16,17,18)/p-2/t4-,6-,7-,8-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3'-O-phosphonatouridine |
|---|
|
Traditional IUPAC Name: |
disodium salt 3'-uridylic acid |
|---|
| SMILES: | C1(=CN(C(=O)NC(=O)1)C2(OC(CO)C(OP(=O)([O-])[O-])C(O)2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as ribonucleoside 3'-phosphates. These are ribonucleosides that contain a phosphate group attached to the C-3 carbon of the ribose or deoxyribose moiety. The nucleobases here are limited to purine, pyrimidine, and pyridine derivatives. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Ribonucleoside 3'-phosphates |
|---|
|
Direct Parent |
Ribonucleoside 3'-phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Pentose monosaccharide
- Hydroxypyrimidine
- Pyrimidone
- Monoalkyl phosphate
- Hydropyrimidine
- Monosaccharide
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Heteroaromatic compound
- Tetrahydrofuran
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Alcohol
- Primary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Thiele I, Swainston N, Fleming RM, Hoppe A, Sahoo S, Aurich MK, Haraldsdottir H, Mo ML, Rolfsson O, Stobbe MD, Thorleifsson SG, Agren R, Bolling C, Bordel S, Chavali AK, Dobson P, Dunn WB, Endler L, Hala D, Hucka M, Hull D, Jameson D, Jamshidi N, Jonsson JJ, Juty N, Keating S, Nookaew I, Le Novere N, Malys N, Mazein A, Papin JA, Price ND, Selkov E Sr, Sigurdsson MI, Simeonidis E, Sonnenschein N, Smallbone K, Sorokin A, van Beek JH, Weichart D, Goryanin I, Nielsen J, Westerhoff HV, Kell DB, Mendes P, Palsson BO: A community-driven global reconstruction of human metabolism. Nat Biotechnol. 2013 Mar 3. doi: 10.1038/nbt.2488. [23455439 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|