|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120041 |
|---|
|
Identification |
|---|
| Name: |
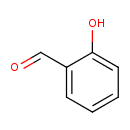
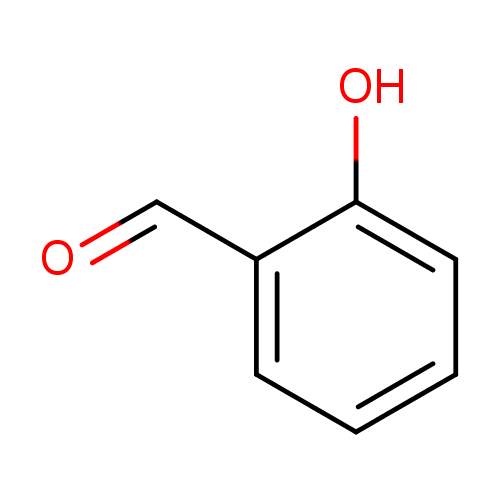
salicylaldehyde |
|---|
| Description: | A hydroxybenzaldehyde carrying a hydroxy substituent at position 2. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-Hydroxybenzaldehyde
- o-formylphenol
- o-Hydroxybenzaldehyde
- salicylal
- Salicylaldehyd
- Salicylaldehyde
- salicylaldehyde
- Salizylaldehyd
|
|---|
|
Chemical Formula: |
C7H6O2 |
|---|
| Average Molecular Weight: |
122.123 |
|---|
| Monoisotopic Molecular
Weight: |
122.03678 |
|---|
| InChI Key: |
SMQUZDBALVYZAC-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C7H6O2/c8-5-6-3-1-2-4-7(6)9/h1-5,9H |
|---|
| CAS
number: |
90-02-8 |
|---|
| IUPAC Name: | 2-hydroxybenzaldehyde |
|---|
|
Traditional IUPAC Name: |
salicylaldehyde |
|---|
| SMILES: | C1(C=CC(O)=C(C=O)C=1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as hydroxybenzaldehydes. These are organic aromatic compounds containing a benzene ring carrying an aldehyde group and a hydroxyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
|
Class |
Benzene and substituted derivatives |
|---|
| Sub Class | Benzaldehydes |
|---|
|
Direct Parent |
Hydroxybenzaldehydes |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydroxybenzaldehyde
- Benzoyl
- Aryl-aldehyde
- Phenol
- Vinylogous acid
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aldehyde
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
0.7 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 0.7 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 17 mg/mL at 86 °C | Not Available | | LogP | 1.81 | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Montalbano F, Cal PM, Carvalho MA, Gonçalves LM, Lucas SD, Guedes RC, Veiros LF, Moreira R, Gois PM (2013)Discovery of new heterocycles with activity against human neutrophile elastase based on a boron promoted one-pot assembly reaction. Organic & biomolecular chemistry 11, Pubmed: 23715243
- Caboni P, Aissani N, Cabras T, Falqui A, Marotta R, Liori B, Ntalli N, Sarais G, Sasanelli N, Tocco G (2013)Potent nematicidal activity of phthalaldehyde, salicylaldehyde, and cinnamic aldehyde against Meloidogyne incognita. Journal of agricultural and food chemistry 61, Pubmed: 23379671
- Kim HK, Yun YK, Ahn YJ (2008)Fumigant toxicity of cassia bark and cassia and cinnamon oil compounds to Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). Experimental & applied acarology 44, Pubmed: 18247142
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|