|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120034 |
|---|
|
Identification |
|---|
| Name: |
L-lactaldehyde |
|---|
| Description: | Not Available |
|---|
|
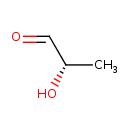
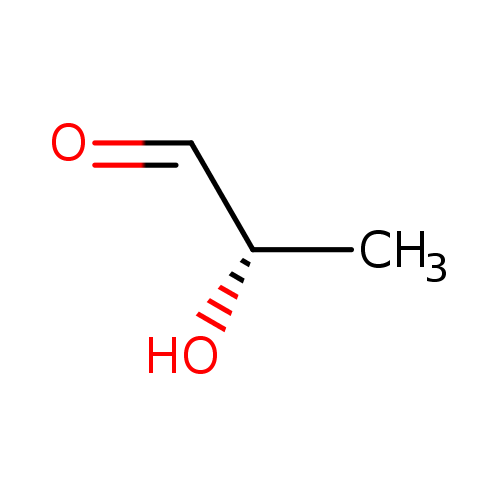
Structure |
|
|---|
| Synonyms: | - (S)-lactaldehyde
- (S)-Lactaldehyde
- (S)-lactaldehyde
- L-2-hydroxypropionaldehyde
- L-2-Hydroxypropionaldehyde
- L-lactaldehyde
- L-Lactaldehyde
|
|---|
|
Chemical Formula: |
C3H6O2 |
|---|
| Average Molecular Weight: |
74.079 |
|---|
| Monoisotopic Molecular
Weight: |
74.03678 |
|---|
| InChI Key: |
BSABBBMNWQWLLU-VKHMYHEASA-N |
|---|
| InChI: | InChI=1S/C3H6O2/c1-3(5)2-4/h2-3,5H,1H3/t3-/m0/s1 |
|---|
| CAS
number: |
598-35-6 |
|---|
| IUPAC Name: | (2S)-2-hydroxypropanal |
|---|
|
Traditional IUPAC Name: |
L-lactaldehyde |
|---|
| SMILES: | CC(C=O)O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as alpha-hydroxyaldehydes. These are organic compounds containing an aldehyde substituted with a hydroxyl group on the adjacent carbon. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Alpha-hydroxyaldehydes |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-hydroxyaldehyde
- Secondary alcohol
- Organic oxide
- Hydrocarbon derivative
- Short-chain aldehyde
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Chen YM, Lin EC: Dual control of a common L-1,2-propanediol oxidoreductase by L-fucose and L-rhamnose in Escherichia coli. J Bacteriol. 1984 Mar;157(3):828-32. [6421801 ]
- Casazza JP, Felver ME, Veech RL: The metabolism of acetone in rat. J Biol Chem. 1984 Jan 10;259(1):231-6. [6706932 ]
- TING SM, SELLINGER OZ, MILLER ON: THE METABOLISM OF LACTALDEHYDE. VI. THE REDUCTION OF D- AND L-LACTALDEHYDE IN RAT LIVER. Biochim Biophys Acta. 1964 Aug 26;89:217-25. [14203169 ]
- TING SM, MILLER ON, SELLINGER OZ: THE METABOLISM OF LACTALDEHYDE. VII. THE OXIDATION OF D-LACTALDEHYDE IN RAT LIVER. Biochim Biophys Acta. 1965 Mar 8;97:407-15. [14323585 ]
- Akhy MT, Brown CM, Old DC: L-Rhamnose utilisation in Salmonella typhimurium. J Appl Bacteriol. 1984 Apr;56(2):269-74. [6373710 ]
- Ros J, Aguilar J: Genetic and structural evidence for the presence of propanediol oxidoreductase isoenzymes in Escherichia coli. J Gen Microbiol. 1984 Mar;130(3):687-92. [6427403 ]
- Di Costanzo L, Gomez GA, Christianson DW: Crystal structure of lactaldehyde dehydrogenase from Escherichia coli and inferences regarding substrate and cofactor specificity. J Mol Biol. 2007 Feb 16;366(2):481-93. Epub 2006 Nov 10. [17173928 ]
- ENGLESBERG E: Physiological basis for rhamnose utilization by a mutant of Pasteurella pestis. I. Experiments with resting cells; the isolation of lactic aldehyde. J Bacteriol. 1957 Jul;74(1):8-11. [13462953 ]
- Chen YM, Chakrabarti T, Lin EC: Constitutive activation of L-fucose genes by an unlinked mutation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):725-9. [6378890 ]
- SANDMAN RP, MILLER ON: Studies on the metabolism of lactaldehyde. I. Separation and determination of lactaldehyde and related 3-carbon compounds. J Biol Chem. 1958 Jan;230(1):353-9. [13502404 ]
|
|---|
| Synthesis Reference: |
Kranz, Cyrill. Synthesis of Lactic Aldehyde. Chemicke Listy pro Vedu a Prumysl (1912), 5 323-7. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|