|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120026 |
|---|
|
Identification |
|---|
| Name: |
caldariellaquinone |
|---|
| Description: | A 1-benzothiophene that is 1-benzothiophene-4,7-dione bearing additional methylthio and 3,7,11,15,19,23-hexamethyltetracosyl substituents at positions 5 and 6 respectively. Isolated from Caldariella acidophila. |
|---|
|
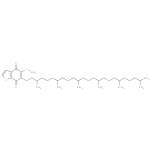
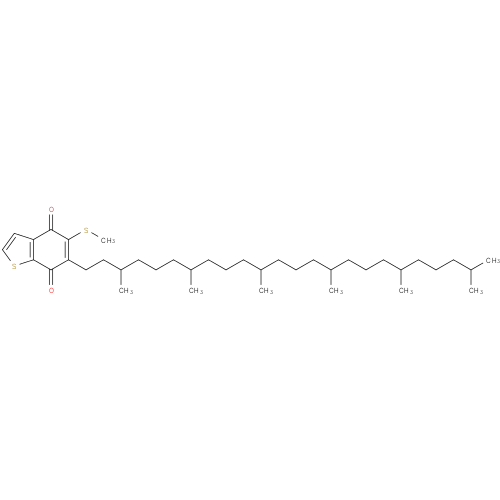
Structure |
|
|---|
| Synonyms: | - 6-(3,7,11,15,19,23-hexamethyltetracosyl)-5-methylthiobenzo[b]thiophen-4,7-quinone
- caldariellaquinone
|
|---|
|
Chemical Formula: |
C39H66O2S2 |
|---|
| Average Molecular Weight: |
631.069 |
|---|
| Monoisotopic Molecular
Weight: |
630.45044 |
|---|
| InChI Key: |
GHRWXPXOBGRSHG-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C39H66O2S2/c1-28(2)14-9-15-29(3)16-10-17-30(4)18-11-19-31(5)20-12-21-32(6)22-13-23-33(7)24-25-34-37(41)39-35(26-27-43-39)36(40)38(34)42-8/h26-33H,9-25H2,1-8H3 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 6-(3,7,11,15,19,23-hexamethyltetracosyl)-5-(methylsulfanyl)-1-benzothiophene-4,7-dione |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC(C)CCCC(C)CCCC(C)CCCC(C)CCCC(C)CCCC(C)CCC2(=C(SC)C(=O)C1(=C(SC=C1)C(=O)2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as sesterterpenoids. These are terpenes composed of five consecutive isoprene units. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Prenol lipids |
|---|
| Sub Class | Sesterterpenoids |
|---|
|
Direct Parent |
Sesterterpenoids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Sesterterpenoid
- Aryl ketone
- Vinylogous thioester
- Heteroaromatic compound
- Thiophene
- Thioenolether
- Ketone
- Organoheterocyclic compound
- Sulfenyl compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
- 1-benzothiophenes, quinone, organic sulfide (CHEBI:73387)
- a quinone (CPD-9612)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Zhou D, White RH (1989)Biosynthesis of caldariellaquinone in Sulfolobus spp. Journal of bacteriology 171, Pubmed: 2512282
- Collins MD, Langworthy TA (1983)Respiratory quinone composition of some acidophilic bacteria. Systematic and applied microbiology 4, Pubmed: 23194730
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|