|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120017 |
|---|
|
Identification |
|---|
| Name: |
5,6-dihydrothymine |
|---|
| Description: | A pyrimidone obtained by formal addition of hydrogen across the 5,6-position of thymine. |
|---|
|
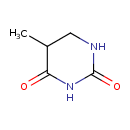
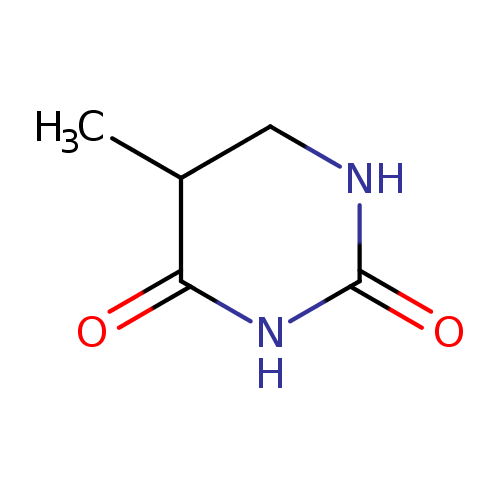
Structure |
|
|---|
| Synonyms: | - 5,6-Dihydro-5-methyluracil
- 5,6-Dihydrothymine
- 5,6-dihydrothymine
- 5-Methyl-5,6-dihydrouracil
- Dihydrothymine
|
|---|
|
Chemical Formula: |
C5H8N2O2 |
|---|
| Average Molecular Weight: |
128.13 |
|---|
| Monoisotopic Molecular
Weight: |
128.05858 |
|---|
| InChI Key: |
NBAKTGXDIBVZOO-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C5H8N2O2/c1-3-2-6-5(9)7-4(3)8/h3H,2H2,1H3,(H2,6,7,8,9) |
|---|
| CAS
number: |
696-04-8 |
|---|
| IUPAC Name: | 5,6-dihydrothymine |
|---|
|
Traditional IUPAC Name: |
dihydrothymine |
|---|
| SMILES: | CC1(C(NC(=O)NC1)=O) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as ureides. These are compounds containing an ureide group with the general structure R1-CO-NH-CO-N(R)2R3, formally derived by the acylation of urea. They can be subdivided in N-acyl or N,N'-diacyl ureas. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Organic carbonic acids and derivatives |
|---|
|
Direct Parent |
Ureides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Ureide
- 5,6-dihydropyrimidine
- Hydropyrimidine
- Azacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Hofmann U, Schwab M, Seefried S, Marx C, Zanger UM, Eichelbaum M, Mürdter TE (2003)Sensitive method for the quantification of urinary pyrimidine metabolites in healthy adults by gas chromatography-tandem mass spectrometry. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 791, Pubmed: 12798197
- Tainaka K, Fujitsuka M, Takada T, Kawai K, Majima T (2010)Sequence dependence of excess electron transfer in DNA. The journal of physical chemistry. B 114, Pubmed: 20509700
- Hubbard K, Ide H, Erlanger BF, Wallace SS (1989)Characterization of antibodies to dihydrothymine, a radiolysis product of DNA. Biochemistry 28, Pubmed: 2669952
|
|---|
| Synthesis Reference: |
Yamane, Tetsuo; Wyluda, Benjamin J.; Shulman, Robert G. Dihydrothymine from ultraviolet-irradiated DNA. Proceedings of the National Academy of Sciences of the United States of America (1967), 58(2), 439-42. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|