|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120011 |
|---|
|
Identification |
|---|
| Name: |
caldariellaquinol |
|---|
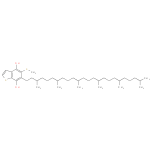
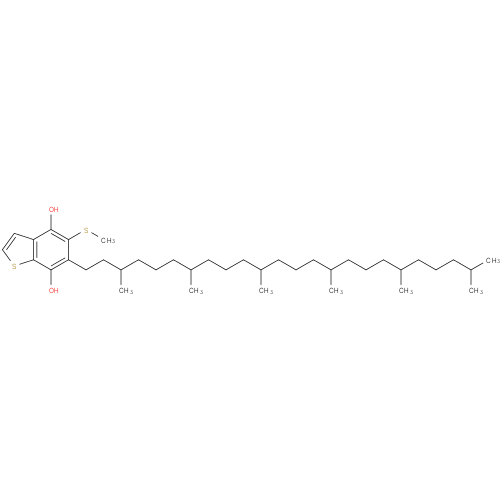
| Description: | A 1-benzothiophene that is 1-benzothiophene-4,7-diol bearing additional methylthio and 3,7,11,15,19,23-hexamethyltetracosyl substituents at positions 5 and 6 respectively |
|---|
|
Structure |
|
|---|
| Synonyms: | Not Available |
|---|
|
Chemical Formula: |
C39H68O2S2 |
|---|
| Average Molecular Weight: |
633.085 |
|---|
| Monoisotopic Molecular
Weight: |
632.46606 |
|---|
| InChI Key: |
UVCQOKDZGIAHDG-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C39H68O2S2/c1-28(2)14-9-15-29(3)16-10-17-30(4)18-11-19-31(5)20-12-21-32(6)22-13-23-33(7)24-25-34-37(41)39-35(26-27-43-39)36(40)38(34)42-8/h26-33,40-41H,9-25H2,1-8H3 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 6-(3,7,11,15,19,23-hexamethyltetracosyl)-5-(methylsulfanyl)-1-benzothiophene-4,7-diol |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC(C)CCCC(C)CCCC(C)CCCC(C)CCCC(C)CCCC(C)CCC2(C(SC)=C(O)C1(=C(SC=C1)C(O)=2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as polyprenylphenols. These are compounds containing a polyisoprene chain attached to a phenol group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Prenol lipids |
|---|
| Sub Class | Polyprenylphenols |
|---|
|
Direct Parent |
Polyprenylphenols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Polyprenylphenol
- Sesterterpenoid
- Prenylbenzoquinol
- Benzothiophene
- 1-benzothiophene
- Aryl thioether
- Thiophenol ether
- Alkylarylthioether
- Benzenoid
- Thiophene
- Heteroaromatic compound
- Sulfenyl compound
- Thioether
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organosulfur compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
- 1-benzothiophenes, organic sulfide, hydroquinones (CHEBI:73388)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Zhou D, White RH (1989)Biosynthesis of caldariellaquinone in Sulfolobus spp. Journal of bacteriology 171, Pubmed: 2512282
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|