|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120006 |
|---|
|
Identification |
|---|
| Name: |
5-hydroxyisourate |
|---|
| Description: | An oxopurine that is 5,7-dihydro-1H-purine-2,6,8(9H)-trione in which the hydrogen at position 5 is substituted by a hydroxy group. |
|---|
|
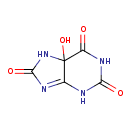
Structure |
|
|---|
| Synonyms: | - 5-Hydroxyisourate
- 5-hydroxyisourate
|
|---|
|
Chemical Formula: |
C5H4N4O4 |
|---|
| Average Molecular Weight: |
184.111 |
|---|
| Monoisotopic Molecular
Weight: |
184.02325 |
|---|
| InChI Key: |
LTQYPAVLAYVKTK-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C5H4N4O4/c10-2-5(13)1(6-3(11)8-2)7-4(12)9-5/h13H,(H3,6,7,8,9,10,11,12) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 5-hydroxy-5,7-dihydro-1H-purine-2,6,8(9H)-trione |
|---|
|
Traditional IUPAC Name: |
5-hydroxyisourate |
|---|
| SMILES: | C2(C1(O)(NC(=O)NC1=NC(=O)N2))(=O) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as alkaloids and derivatives. These are naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and more rarely other elements such as chlorine, bromine, and phosphorus. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
|
Class |
Not Available |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Alkaloids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alkaloid or derivatives
- Xanthine
- Purinone
- Purine
- Ureide
- Pyrimidone
- Pyrimidine
- 1,3-diazinane
- 3-imidazoline
- Urea
- Carboxamide group
- Azacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Carboxylic acid derivative
- Carboxylic acid amidine
- Amidine
- Alkanolamine
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Afendi FM, Okada T, Yamazaki M, Hirai-Morita A, Nakamura Y, Nakamura K, Ikeda S, Takahashi H, Altaf-Ul-Amin M, Darusman LK, Saito K, Kanaya S. (2012) KNApSAcK family databases: integrated metabolite-plant species databases for multifaceted plant research. Plant Cell Physiol. 2012 Feb;53(2):e1.
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|