|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110836 |
|---|
|
Identification |
|---|
| Name: |
hydrogen sulfite |
|---|
| Description: | The hydrogen sulfite, or bisulfite, ion is the ion HSO3-. It is the conjugate base of sulfurous acid, H2SO3. Bisulfite has long been recognized as a reagent to react with organic compounds in various ways; prominent among them are additions to carbonyl groups and to carbon-carbon double bonds, and free radical reactions in the presence of oxygen. Bisulfite reacts with pyrimidine nucleosides, undergoing additions to the 5,6-double bond to form pyrimidine- 5,6-dihydro-6-sulfonates. The addition across the 5,6-double bond is reversible. All these adducts are unstable in alkali. Bisulfite modification has been used to probe secondary or higher structures of polynucleotides as it reacts with pyrimidines in single-stranded regions specifically. In animal DNA, a portion of the pyrimidine base cytosine is methylated at position 5. 5-Methylcytosine in DNA is now an intensive focus of attention for its roles in gene functions. The methylation occurs by postreplication modification, and is a heritable event. 5-Methylcytosine sites are known to be mutation hot spots. 5-Methylcytosine spontaneously deaminates into thymine, while cytosine does so only more slowly. Determination of the position of 5-methylcytosine in a given DNA requires some means to distinguish 5- methylcytosine from cytosine. Chemical modification can be used as one such means. Treatment of DNA with bisulfite converts cytosine into uracil by deamination, while 5-methylcytosine remains mostly unaltered. The majority of recent research on 5-methylcytosine in DNA employs bisulfite treatment in the analytical procedure. The principle of this procedure is as follows. As uracil is a thymine-analog (5-methyluracil is thymine), it behaves toward DNA polymerases as thymine. When the bisulfite-modified DNA is subjected to PCR (Polymerase Chain Reaction), a process necessary to amplify tiny samples of DNA, the uracil residues will become thymine residues in the amplified products. As 5-methylcytosine residues in the original DNA sample remain unaltered during the bisulfite treatment, the amplification will produce polynucleotides in which cytosine residues represent the 5-methylcytosine residues of the original. (Genes and Environment (2006), 28(1), 1-8.). |
|---|
|
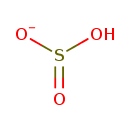
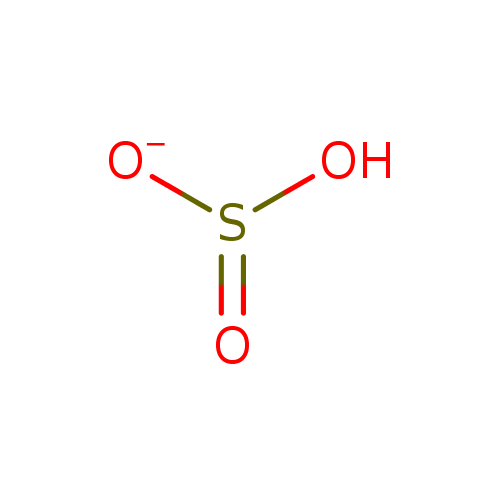
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
HO3S
|
|---|
| Average Molecular Weight: |
81.9724646205 |
|---|
| Monoisotopic Molecular
Weight: |
81.9724646205 |
|---|
| InChI Key: |
LSNNMFCWUKXFEE-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/H2O3S/c1-4(2)3/h(H2,1,2,3)/p-1 |
|---|
| CAS
number: |
15181-46-1 |
|---|
| IUPAC Name: | hydrogen(trioxidosulfate)(1−) |
|---|
|
Traditional IUPAC Name: |
bisulfite |
|---|
| SMILES: | OS(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as non-metal sulfites. These are inorganic non-metallic compounds containing a sulfite as its largest oxoanion. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Inorganic compounds |
|---|
|
Class |
Homogeneous non-metal compounds |
|---|
| Sub Class | Non-metal oxoanionic compounds |
|---|
|
Direct Parent |
Non-metal sulfites |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Non-metal sulfite
- Inorganic oxide
|
|---|
| Molecular Framework |
Not Available |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Wilkinson, Peter M.; Doldersum, Bert; Cramers, Peter H. M. R.; Van Dierendonck, Laurent L. The kinetics of uncatalyzed sodium sulfite oxidation. Chemical Engineering Science (1993), 48(5), 933-41. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|