|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110835 |
|---|
|
Identification |
|---|
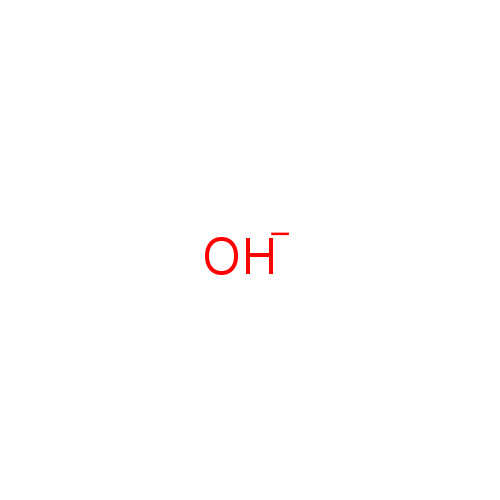
| Name: |
OH- |
|---|
| Description: | In chemistry, hydroxide is the most common name for the diatomic anion OH, consisting of oxygen and hydrogen atoms, usually derived from the dissociation of a base. It is one of the simplest diatomic ions known. Hydroxide ion is a kind of ligand. It donates one pair of electrons, behaving as a Lewis base. Examples include the aluminate ion [Al(OH)4]- and aurate ion [Au(OH)4]-. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
hydroxyl
-
hydroxyl ion
-
OH
-
hydroxide
-
hydroxide ion
|
|---|
|
Chemical Formula: |
HO
|
|---|
| Average Molecular Weight: |
18.0105646863 |
|---|
| Monoisotopic Molecular
Weight: |
18.0105646863 |
|---|
| InChI Key: |
XLYOFNOQVPJJNP-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/H2O/h1H2/p-1 |
|---|
| CAS
number: |
14280-30-9 |
|---|
| IUPAC Name: | hydridooxygenate(1−) |
|---|
|
Traditional IUPAC Name: |
hydroxide |
|---|
| SMILES: | [O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as non-metal hydroxides. These are inorganic non-metallic compounds containing the hydroxide group as its largest oxoanion. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Inorganic compounds |
|---|
|
Class |
Homogeneous non-metal compounds |
|---|
| Sub Class | Non-metal oxoanionic compounds |
|---|
|
Direct Parent |
Non-metal hydroxides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Non-metal hydroxide
- Inorganic hydride
- Inorganic oxide
|
|---|
| Molecular Framework |
Not Available |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Revzin, G. E.; Lavrent'eva, V. G.; Revzina, T. V.; Kashina, N. I. Preparation of hydroxides slightly soluble in water. U.S.S.R. (1967), CODEN: URXXAF SU 196735 19670531 CAN 68:4526 AN 1968:4526 |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|