|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110825 |
|---|
|
Identification |
|---|
| Name: |
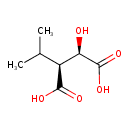
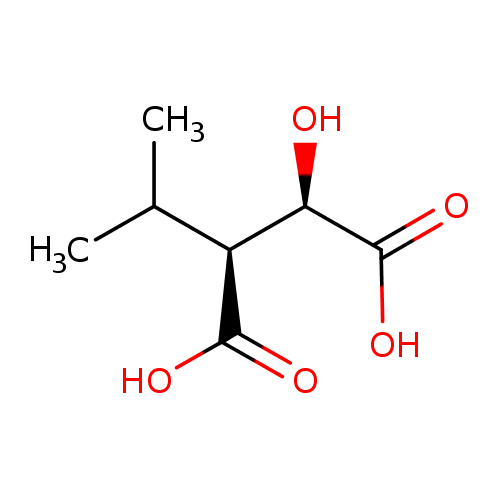
(2R,3S)-3-isopropylmalate |
|---|
| Description: | 3-Isopropylmalate is an intermediate in valine, leucine and isoleucine biosynthesis. It is a substrate for 3-isopropylmalate dehydrogenase (TT_C0867) and can be generated from the reduction of 2-isopropyl-3-oxosuccinate. Leucine biosynthesis involves a five-step conversion process starting with the valine precursor 2-keto-isovalerate. The final step in this pathway is catalyzed by two transaminases of broad specificity, Branched-chain amino acid transferase (IlvE) and Tyrosine aminotransferase (TyrB). This pathway is part of the superpathway of leucine, valine, and isoleucine biosynthesis, that generates not only isoleucine and valine, but also leucine. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
2-D-threo-hydroxy-3-carboxy-isocaproate
-
3-carboxy-2-hydroxy-4-methylpentanoate
-
β-isopropylmalate
-
3-isopropylmalate
|
|---|
|
Chemical Formula: |
C7H10O5
|
|---|
| Average Molecular Weight: |
174.15 |
|---|
| Monoisotopic Molecular
Weight: |
176.0684734957 |
|---|
| InChI Key: |
RNQHMTFBUSSBJQ-CRCLSJGQSA-L |
|---|
| InChI: |
InChI=1S/C7H12O5/c1-3(2)4(6(9)10)5(8)7(11)12/h3-5,8H,1-2H3,(H,9,10)(H,11,12)/p-2/t4-,5+/m0/s1 |
|---|
| CAS
number: |
921-28-8 |
|---|
| IUPAC Name: | (2R,3S)-2-hydroxy-3-(propan-2-yl)butanedioate |
|---|
|
Traditional IUPAC Name: |
(2R,3S)-3-isopropylmalic acid |
|---|
| SMILES: | CC(C)C(C([O-])=O)C(C([O-])=O)O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as hydroxy fatty acids. These are fatty acids in which the chain bears a hydroxyl group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Lipids and lipid-like molecules |
|---|
| Sub Class | Fatty Acyls |
|---|
|
Direct Parent |
Hydroxy fatty acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Beta-hydroxy acid
- Branched fatty acid
- Hydroxy fatty acid
- Short-chain hydroxy acid
- Methyl-branched fatty acid
- Alpha-hydroxy acid
- Hydroxy acid
- Dicarboxylic acid or derivatives
- Secondary alcohol
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|