|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110822 |
|---|
|
Identification |
|---|
| Name: |
acetyl phosphate |
|---|
| Description: | Acetylphosphate or actyl phosphate is a compound involved in taurine and hypotaurine metabolism as well as pyruvate metabolism. It is generated from sulfoacetaldehyde, converted to acetyl-CoA and acetate via phosphate acetyltransferase (EC:2.3.1.8) and acetate kinase (EC:2.7.2.1) respectively. It is also an intermediate in pyruvate metabolism. It is generated from pyruvate and the formation is catalyzed by pyruvate oxidase (EC:1.2.3.3). |
|---|
|
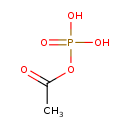
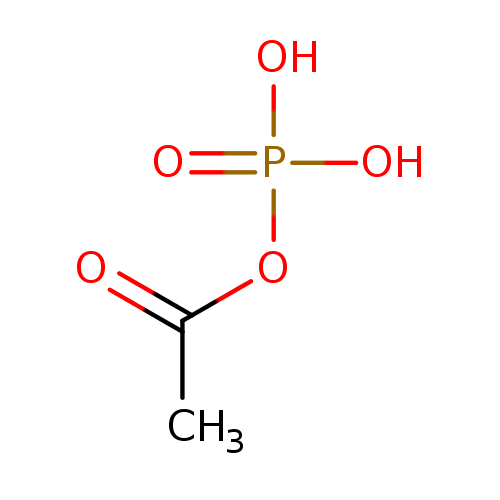
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C2H3O5P
|
|---|
| Average Molecular Weight: |
138.02 |
|---|
| Monoisotopic Molecular
Weight: |
139.987459781 |
|---|
| InChI Key: |
LIPOUNRJVLNBCD-UHFFFAOYSA-L |
|---|
| InChI: |
InChI=1S/C2H5O5P/c1-2(3)7-8(4,5)6/h1H3,(H2,4,5,6)/p-2 |
|---|
| CAS
number: |
590-54-5 |
|---|
| IUPAC Name: | acetyl phosphate |
|---|
|
Traditional IUPAC Name: |
acetylphosphate |
|---|
| SMILES: | CC(OP([O-])(=O)[O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as acyl monophosphates. These are organic compounds containing a monophosphate linked to an acyl group. They have the general structure R-CO-P(O)(O)OH, R=H or organyl. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Organic phosphoric acids and derivatives |
|---|
| Sub Class | Phosphate esters |
|---|
|
Direct Parent |
Acyl monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Acyl monophosphate
- Acetate salt
- Carboxylic acid salt
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organic salt
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Verdin E, Ott M (2013)Acetylphosphate: a novel link between lysine acetylation and intermediary metabolism in bacteria. Molecular cell 51, Pubmed: 23870140
- Kuhn ML, Zemaitaitis B, Hu LI, Sahu A, Sorensen D, Minasov G, Lima BP, Scholle M, Mrksich M, Anderson WF, Gibson BW, Schilling B, Wolfe AJ (2014)Structural, kinetic and proteomic characterization of acetyl phosphate-dependent bacterial protein acetylation. PloS one 9, Pubmed: 24756028
|
|---|
| Synthesis Reference: |
Costakel, O.; Kitzoulesco, I. The possibility of acetylphosphate synthesis in cancerous and noncancerous human serum at pH 4. Rev. sci. Med., Acad. rep. populaire Roumaine (1960), 5 7-10. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|