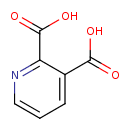
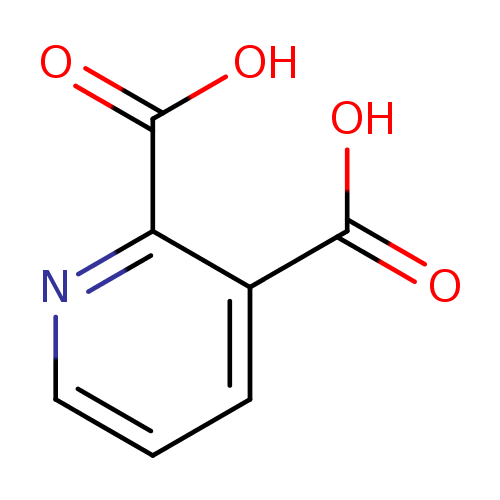
| References: |
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- Shoemaker JD, Elliott WH: Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991 Jan 2;562(1-2):125-38. [2026685 ]
- Guneral F, Bachmann C: Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin Chem. 1994 Jun;40(6):862-6. [8087979 ]
- Medana IM, Hien TT, Day NP, Phu NH, Mai NT, Chu'ong LV, Chau TT, Taylor A, Salahifar H, Stocker R, Smythe G, Turner GD, Farrar J, White NJ, Hunt NH: The clinical significance of cerebrospinal fluid levels of kynurenine pathway metabolites and lactate in severe malaria. J Infect Dis. 2002 Mar 1;185(5):650-6. Epub 2002 Feb 14. [11865422 ]
- Smythe GA, Poljak A, Bustamante S, Braga O, Maxwell A, Grant R, Sachdev P: ECNI GC-MS analysis of picolinic and quinolinic acids and their amides in human plasma, CSF, and brain tissue. Adv Exp Med Biol. 2003;527:705-12. [15206793 ]
- Medana IM, Day NP, Salahifar-Sabet H, Stocker R, Smythe G, Bwanaisa L, Njobvu A, Kayira K, Turner GD, Taylor TE, Hunt NH: Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J Infect Dis. 2003 Sep 15;188(6):844-9. Epub 2003 Sep 9. [12964115 ]
- Heyes MP, Achim CL, Wiley CA, Major EO, Saito K, Markey SP: Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem J. 1996 Dec 1;320 ( Pt 2):595-7. [8973572 ]
- Heyes MP, Saito K, Lackner A, Wiley CA, Achim CL, Markey SP: Sources of the neurotoxin quinolinic acid in the brain of HIV-1-infected patients and retrovirus-infected macaques. FASEB J. 1998 Jul;12(10):881-96. [9657528 ]
- Heyes MP, Saito K, Milstien S, Schiff SJ: Quinolinic acid in tumors, hemorrhage and bacterial infections of the central nervous system in children. J Neurol Sci. 1995 Nov;133(1-2):112-8. [8583213 ]
- Wolfensberger M, Amsler U, Cuenod M, Foster AC, Whetsell WO Jr, Schwarcz R: Identification of quinolinic acid in rat and human brain tissue. Neurosci Lett. 1983 Nov 11;41(3):247-52. [6664615 ]
- Kurup RK, Kurup PA: Hypothalamic digoxin, hemispheric chemical dominance, and Alzheimer's disease. Int J Neurosci. 2003 Mar;113(3):361-81. [12803139 ]
- Heyes MP, Wyler AR, Devinsky O, Yergey JA, Markey SP, Nadi NS: Quinolinic acid concentrations in brain and cerebrospinal fluid of patients with intractable complex partial seizures. Epilepsia. 1990 Mar-Apr;31(2):172-7. [1690639 ]
- Kurup RK, Kurup PA: Central role of hypothalamic digoxin in conscious perception, neuroimmunoendocrine integration, and coordination of cellular function: relation to hemispheric dominance. Int J Neurosci. 2002 Jun;112(6):705-39. [12325312 ]
- Moroni F, Lombardi G, Carla V, Lal S, Etienne P, Nair NP: Increase in the content of quinolinic acid in cerebrospinal fluid and frontal cortex of patients with hepatic failure. J Neurochem. 1986 Dec;47(6):1667-71. [2430055 ]
- Basile AS, Saito K, al-Mardini H, Record CO, Hughes RD, Harrison P, Williams R, Li Y, Heyes MP: The relationship between plasma and brain quinolinic acid levels and the severity of hepatic encephalopathy. Gastroenterology. 1995 Mar;108(3):818-23. [7875484 ]
- Power C, Johnson RT: HIV-1 associated dementia: clinical features and pathogenesis. Can J Neurol Sci. 1995 May;22(2):92-100. [7627922 ]
- Ellison DW, Beal MF, Mazurek MF, Malloy JR, Bird ED, Martin JB: Amino acid neurotransmitter abnormalities in Huntington's disease and the quinolinic acid animal model of Huntington's disease. Brain. 1987 Dec;110 ( Pt 6):1657-73. [2892568 ]
- Saito K, Markey SP, Heyes MP: Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience. 1992 Nov;51(1):25-39. [1465184 ]
- Heyes MP, Rubinow D, Lane C, Markey SP: Cerebrospinal fluid quinolinic acid concentrations are increased in acquired immune deficiency syndrome. Ann Neurol. 1989 Aug;26(2):275-7. [2528321 ]
- Heyes MP, Ellis RJ, Ryan L, Childers ME, Grant I, Wolfson T, Archibald S, Jernigan TL: Elevated cerebrospinal fluid quinolinic acid levels are associated with region-specific cerebral volume loss in HIV infection. Brain. 2001 May;124(Pt 5):1033-42. [11335705 ]
- Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, Dilling LA, Elia J, Kruesi MJ, Lackner A, et al.: Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992 Oct;115 ( Pt 5):1249-73. [1422788 ]
|
|---|