|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110805 |
|---|
|
Identification |
|---|
| Name: |
uroporphyrinogen-III |
|---|
| Description: | An octacarboxylic acid anion obtained by deprotonation of all eight carboxy groups of uroporphyrinogen III. |
|---|
|
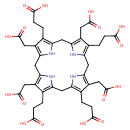
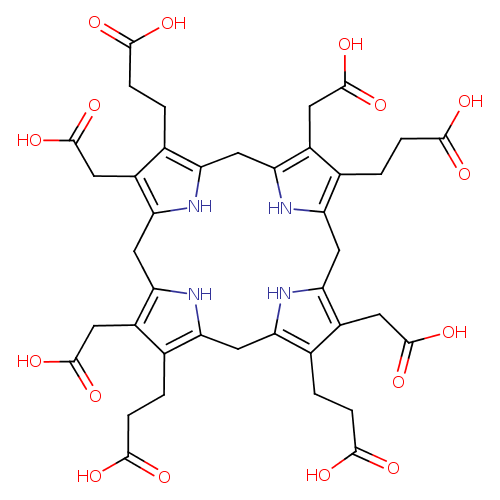
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C40H36N4O16
|
|---|
| Average Molecular Weight: |
828.74 |
|---|
| Monoisotopic Molecular
Weight: |
836.2752313868 |
|---|
| InChI Key: |
HUHWZXWWOFSFKF-UHFFFAOYSA-F |
|---|
| InChI: |
InChI=1S/C40H44N4O16/c45-33(46)5-1-17-21(9-37(53)54)29-14-27-19(3-7-35(49)50)22(10-38(55)56)30(43-27)15-28-20(4-8-36(51)52)24(12-40(59)60)32(44-28)16-31-23(11-39(57)58)18(2-6-34(47)48)26(42-31)13-25(17)41-29/h41-44H,1-16H2,(H,45,46)(H,47,48)(H,49,50)(H,51,52)(H,53,54)(H,55,56)(H,57,58)(H,59,60)/p-8 |
|---|
| CAS
number: |
1976-85-8 |
|---|
| IUPAC Name: | 3,3',3'',3'''- [3,8,13,17- [3,8,13,17- tetrakis(carboxylatomethyl)- tetrakis(carboxylatomethyl)- 5,10,15,20,22,24- 5,10,15,20,22,24- hexahydroporphyrin- hexahydroporphyrin- 2,7,12,18- 2,7,12,18- tetrayl]tetrapropanoate tetrayl]tetrapropanoate |
|---|
|
Traditional IUPAC Name: |
uroporphyrinogen-III |
|---|
| SMILES: | C(=O)([O-])CCC3(C(=C2(CC5(NC(CC4(NC(CC1(NC(=C(C=1CC(=O)[O-])CCC(=O)[O-])CC(N2)=3))=C(CC(=O)[O-])C=4CCC(=O)[O-]))=C(CC([O-])=O)C(CCC(=O)[O-])=5)))CC(=O)[O-]) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as porphyrins. These are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Tetrapyrroles and derivatives |
|---|
|
Direct Parent |
Porphyrins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Porphyrin
- Substituted pyrrole
- Pyrrole
- Heteroaromatic compound
- Carboxylic acid derivative
- Carboxylic acid
- Azacycle
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -8 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Aizencang G, Solis C, Bishop DF, Warner C, Desnick RJ: Human uroporphyrinogen-III synthase: genomic organization, alternative promoters, and erythroid-specific expression. Genomics. 2000 Dec 1;70(2):223-31. [11112350 ]
- Martins BM, Grimm B, Mock HP, Huber R, Messerschmidt A: Crystal structure and substrate binding modeling of the uroporphyrinogen-III decarboxylase from Nicotiana tabacum. Implications for the catalytic mechanism. J Biol Chem. 2001 Nov 23;276(47):44108-16. Epub 2001 Aug 27. [11524417 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|