|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110804 |
|---|
|
Identification |
|---|
| Name: |
protoporphyrinogen IX |
|---|
| Description: | Dicarboxylate anion of protoporphyrinogen. |
|---|
|
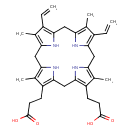
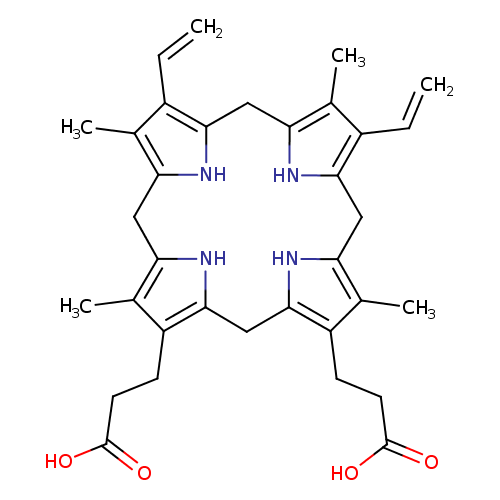
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C34H38N4O4
|
|---|
| Average Molecular Weight: |
566.7 |
|---|
| Monoisotopic Molecular
Weight: |
568.3049557932 |
|---|
| InChI Key: |
UHSGPDMIQQYNAX-UHFFFAOYSA-L |
|---|
| InChI: |
InChI=1S/C34H40N4O4/c1-7-21-17(3)25-13-26-19(5)23(9-11-33(39)40)31(37-26)16-32-24(10-12-34(41)42)20(6)28(38-32)15-30-22(8-2)18(4)27(36-30)14-29(21)35-25/h7-8,35-38H,1-2,9-16H2,3-6H3,(H,39,40)(H,41,42)/p-2 |
|---|
| CAS
number: |
7412-77-3 |
|---|
| IUPAC Name: | 3,3'- (8,13- (8,13- diethynyl- diethynyl- 3,7,12,17- 3,7,12,17- tetramethyl- tetramethyl- 5,10,15,20,22,24- 5,10,15,20,22,24- hexahydroporphyrin- hexahydroporphyrin- 2,18- 2,18- diyl)dipropanoate diyl)dipropanoate |
|---|
|
Traditional IUPAC Name: |
protoporphyrinogen |
|---|
| SMILES: | C=CC1(=C5(NC(=C1C)CC2(=C(C(=C(N2)CC3(NC(=C(C=3CCC([O-])=O)C)CC4(=C(C(=C(N4)C5)C)C=C)))CCC(=O)[O-])C))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as porphyrins. These are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Tetrapyrroles and derivatives |
|---|
|
Direct Parent |
Porphyrins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Porphyrin
- Dicarboxylic acid or derivatives
- Substituted pyrrole
- Pyrrole
- Heteroaromatic compound
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Wan J, Zhang L, Yang G, Zhan CG: Quantitative structure-activity relationship for cyclic imide derivatives of protoporphyrinogen oxidase inhibitors: a study of quantum chemical descriptors from density functional theory. J Chem Inf Comput Sci. 2004 Nov-Dec;44(6):2099-105. [15554680 ]
- Herrick AL, Moore MR, Thompson GG, Ford GP, McColl KE: Cholelithiasis in patients with variegate porphyria. J Hepatol. 1991 Jan;12(1):50-3. [2007776 ]
- Krijt J, Stranska P, Maruna P, Vokurka M, Sanitrak J: Herbicide-induced experimental variegate porphyria in mice: tissue porphyrinogen accumulation and response to porphyrogenic drugs. Can J Physiol Pharmacol. 1997 Oct-Nov;75(10-11):1181-7. [9431441 ]
- Bloomer JR: Enzyme defects in the porphyrias and their relevance to the biochemical abnormalities in these disorders. J Invest Dermatol. 1981 Jul;77(1):102-6. [7252240 ]
- de Villiers JN, Kotze MJ, van Heerden CJ, Sadie A, Gardner HF, Liebenberg J, van Zyl R, du Plessis L, Kimberg M, Frank J, Warnich L: Overrepresentation of the founder PPOX gene mutation R59W in a South African patient with severe clinical manifestation of porphyria. Exp Dermatol. 2005 Jan;14(1):50-5. [15660919 ]
- Corrigall AV, Hift RJ, Adams PA, Kirsch RE: Inhibition of mammalian protoporphyrinogen oxidase by acifluorfen. Biochem Mol Biol Int. 1994 Dec;34(6):1283-9. [7697001 ]
- Cox TM, Jack N, Lofthouse S, Watling J, Haines J, Warren MJ: King George III and porphyria: an elemental hypothesis and investigation. Lancet. 2005 Jul 23-29;366(9482):332-5. [16039338 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|