|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110802 |
|---|
|
Identification |
|---|
| Name: |
uroporphyrinogen-I |
|---|
| Description: | The cyclic tetrapyrrole anion that is the octacarboxylate anion of uroporphyrinogen I. |
|---|
|
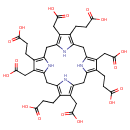
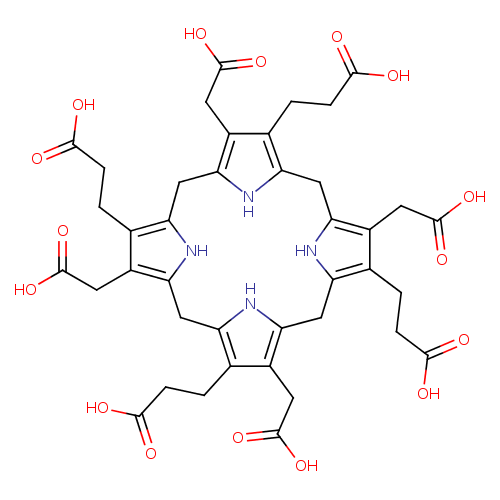
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C40H36N4O16
|
|---|
| Average Molecular Weight: |
836.2752313868 |
|---|
| Monoisotopic Molecular
Weight: |
836.2752313868 |
|---|
| InChI Key: |
QTTNOSKSLATGQB-UHFFFAOYSA-F |
|---|
| InChI: |
InChI=1S/C40H44N4O16/c45-33(46)5-1-17-21(9-37(53)54)29-14-26-19(3-7-35(49)50)23(11-39(57)58)31(43-26)16-28-20(4-8-36(51)52)24(12-40(59)60)32(44-28)15-27-18(2-6-34(47)48)22(10-38(55)56)30(42-27)13-25(17)41-29/h41-44H,1-16H2,(H,45,46)(H,47,48)(H,49,50)(H,51,52)(H,53,54)(H,55,56)(H,57,58)(H,59,60)/p-8 |
|---|
| CAS
number: |
1867-62-5 |
|---|
| IUPAC Name: | 3,3',3'',3'''- [3,8,13,18- [3,8,13,18- tetrakis(carboxylatomethyl)- tetrakis(carboxylatomethyl)- 5,10,15,20,22,24- 5,10,15,20,22,24- hexahydroporphyrin- hexahydroporphyrin- 2,7,12,17- 2,7,12,17- tetrayl]tetrapropanoate tetrayl]tetrapropanoate |
|---|
|
Traditional IUPAC Name: |
uroporphyrinogen I |
|---|
| SMILES: | C(=O)([O-])CCC5(=C4(NC(CC1(NC(=C(CC([O-])=O)C(CCC(=O)[O-])=1)CC2(NC(=C(C(CCC(=O)[O-])=2)CC(=O)[O-])CC3(=C(CCC([O-])=O)C(CC(=O)[O-])=C(N3)C4))))=C(CC(=O)[O-])5)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as porphyrins. These are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Tetrapyrroles and derivatives |
|---|
|
Direct Parent |
Porphyrins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Porphyrin
- Substituted pyrrole
- Pyrrole
- Heteroaromatic compound
- Carboxylic acid derivative
- Carboxylic acid
- Azacycle
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -8 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Maines MD, Mayer RD: Inhibition of testicular cytochrome P-450-dependent steroid biosynthesis by cis-platinum. Reversal by human chorionic gonadotropin. J Biol Chem. 1985 May 25;260(10):6063-8. [4039724 ]
- Mukerji SK, Pimstone NR: Defective human erythrocyte uroporphyrinogen decarboxylase in familial porphyria cutanea tarda: the metabolic lesion or the result of endogenous porphyrinemia? Biochem Biophys Res Commun. 1988 Jul 15;154(1):39-46. [3395340 ]
|
|---|
| Synthesis Reference: |
Burton, Gerardo; Fagerness, Paul E.; Hosozawa, Shigeki; Jordan, Peter M.; Scott, A. Ian. Carbon-13 NMR evidence for a new intermediate, pre-uroporphyrinogen, in the enzymic transformation of porphobilinogen into uroporphyrinogens I and III. Journal of the Chemical Society, Chemical Communications (1979), (5), 202-4. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|