|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110782 |
|---|
|
Identification |
|---|
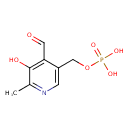
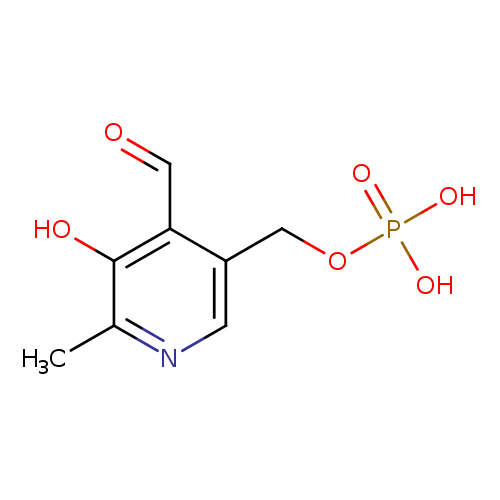
| Name: |
pyridoxal 5'-phosphate |
|---|
| Description: | The dianion resulting from the removal of two protons from the phosphate group of pyridoxal 5'-phosphate. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
PLP
-
pyridoxal phosphate
-
pyridoxal-5P
-
pyridoxal 5-phosphate
-
pyridoxal-P
-
vitamin B6
|
|---|
|
Chemical Formula: |
C8H8NO6P
|
|---|
| Average Molecular Weight: |
245.13 |
|---|
| Monoisotopic Molecular
Weight: |
247.0245735688 |
|---|
| InChI Key: |
NGVDGCNFYWLIFO-UHFFFAOYSA-L |
|---|
| InChI: |
InChI=1S/C8H10NO6P/c1-5-8(11)7(3-10)6(2-9-5)4-15-16(12,13)14/h2-3,11H,4H2,1H3,(H2,12,13,14)/p-2 |
|---|
| CAS
number: |
54-47-7 |
|---|
| IUPAC Name: | (4-formyl-5-hydroxy-6-methylpyridin-3-yl)methyl phosphate |
|---|
|
Traditional IUPAC Name: |
pyridoxal phosphate |
|---|
| SMILES: | CC1(N=CC(=C(C=1O)C=O)COP(=O)([O-])[O-]) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pyridoxals and derivatives. These are compounds containing a pyridoxal moiety, which consists of a pyridine ring substituted at positions 2,3,4, and 5 by a methyl group, a hydroxyl group, a carbaldehyde group, and a hydroxymethyl group, respectively. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Pyridines and derivatives |
|---|
|
Direct Parent |
Pyridoxals and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyridoxal
- Aryl-aldehyde
- Monoalkyl phosphate
- Hydroxypyridine
- Methylpyridine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Vinylogous acid
- Heteroaromatic compound
- Azacycle
- Organopnictogen compound
- Aldehyde
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
255 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 255 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 28 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 3 TMS) | splash10-0gb9-2690000000-c9bacb7e657461e28407 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0f6t-0690000000-4aa57f3f28f0bdef9dcf | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0fxx-8900000000-ee972556340c61650528 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-014l-9100000000-3cc29fb51820adecd59c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0002-0090000000-9b86e80a9dd0de14ab59 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-03dj-9800000000-9313aa9cbf19bc6311c5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0002-9500000000-7ce120a58879e2214ce5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-004j-9200000000-7e6bd8c298613e59fb5d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-004i-9000000000-4c05178b1645bf01f3fa | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0udi-3920000000-6c5e6106f5658d1d6c50 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0udi-3920000000-f96097815e6922356131 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-002b-9000000000-2c4e243699a95e3a0f88 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-002b-9000000000-8aa111485891af9f0d02 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f6t-1790000000-1dcb07cd1110c71f7005 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900000000-ca6aab7f31a36863a417 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9800000000-053802b9c0533d7943ea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9080000000-12ca63d34d39d59f6b42 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-144099ec201adbbc4684 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-70a9559d65e78c488e7e | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Neres J, Brewer ML, Ratier L, Botti H, Buschiazzo A, Edwards PN, Mortenson PN, Charlton MH, Alzari PM, Frasch AC, Bryce RA, Douglas KT (2009)Discovery of novel inhibitors of Trypanosoma cruzi trans-sialidase from in silico screening. Bioorganic & medicinal chemistry letters 19, Pubmed: 19144516
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|