|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110781 |
|---|
|
Identification |
|---|
| Name: |
pyridoxamine |
|---|
| Description: | An ammonium ion that is the conjugate acid of pyridoxamine arising from selective protonation of the primary amino group; major species at pH 7.3. |
|---|
|
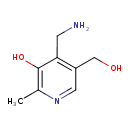
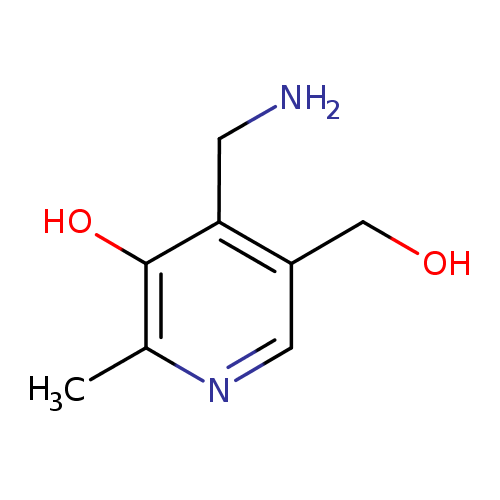
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C8H13N2O2
|
|---|
| Average Molecular Weight: |
169.2 |
|---|
| Monoisotopic Molecular
Weight: |
169.0977026719 |
|---|
| InChI Key: |
NHZMQXZHNVQTQA-UHFFFAOYSA-O |
|---|
| InChI: |
InChI=1S/C8H12N2O2/c1-5-8(12)7(2-9)6(4-11)3-10-5/h3,11-12H,2,4,9H2,1H3/p+1 |
|---|
| CAS
number: |
85-87-0 |
|---|
| IUPAC Name: | [3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl]methanaminium |
|---|
|
Traditional IUPAC Name: |
pyridoxamine |
|---|
| SMILES: | CC1(=NC=C(CO)C(C[N+])=C(O)1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pyridoxamine 5'-phosphates. These are heterocyclic aromatic compounds containing a pyridoxamine that carries a phosphate group at the 5'-position. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Pyridines and derivatives |
|---|
| Sub Class | Pyridoxamines |
|---|
|
Direct Parent |
Pyridoxamine 5'-phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyridoxamine 5'-phosphate
- Aralkylamine
- Hydroxypyridine
- Methylpyridine
- Heteroaromatic compound
- Azacycle
- Amine
- Hydrocarbon derivative
- Alcohol
- Aromatic alcohol
- Primary amine
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | +1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 815 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-001j-0590000000-a4bf9fe291241c903e3d | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-9350000000-3b1415672e7ab22d5e64 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-001i-1490000000-7214a8743cbe260268ce | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-014i-0900000000-4ca97fcd886d80336a5c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-001i-1900000000-499c48bce709456248bc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0059-9200000000-aee74b4498be7c8cea6f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-014i-0900000000-f1da14360063a04c11b4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-014r-0900000000-f5aea0fa928eb266a79d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-00di-0900000000-bebad702dbdba57d61fa | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-05fr-0900000000-7322846ca1b4d95b5b16 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0ab9-2900000000-da43b1a049d349d91831 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-014i-0900000000-6b5f1d606df91ae52eb4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive | splash10-0gb9-0900000000-d5a16a4cdc19453ae9c7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-01b9-0900000000-3a1f853ce36bb047e05f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0900000000-a1db882596b08c2987a7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-0900000000-b0f20e8503b963c8a31f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-3900000000-b41efa32a572817ef0c9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-cd842fcc776d552a0f07 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-0900000000-ae76358c88e1a1cf17dc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0596-9600000000-eeec53c65ada2bc944b0 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- Esteve-Romero J, Capella-Peiro ME, Monferrer-Pons L, Gil-Agusti M: Micellar liquid chromatography in clinical chemistry: application to the monitorization of B6 vitamins. Clin Chim Acta. 2004 Oct;348(1-2):69-77. [15369738 ]
- Berzas Nevado JJ, Murillo Pulgarin JA, Gomez Laguna MA: Determination of pyridoxamine in urine by matrix isopotential synchronous fluorescence spectrometry. Analyst. 1995 Jan;120(1):171-4. [7710125 ]
- Sharma SK, Dakshinamurti K: Determination of vitamin B6 vitamers and pyridoxic acid in biological samples. J Chromatogr. 1992 Jul 1;578(1):45-51. [1400785 ]
- Rokitzki L, Sagredos AN, Reuss F, Buchner M, Keul J: Acute changes in vitamin B6 status in endurance athletes before and after a marathon. Int J Sport Nutr. 1994 Jun;4(2):154-65. [8054960 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|