|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110780 |
|---|
|
Identification |
|---|
| Name: |
pyridoxamine 5'-phosphate |
|---|
| Description: | An organophosphate oxoanion that is the conjugate base of pyridoxamine 5'-phosphate. |
|---|
|
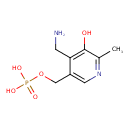
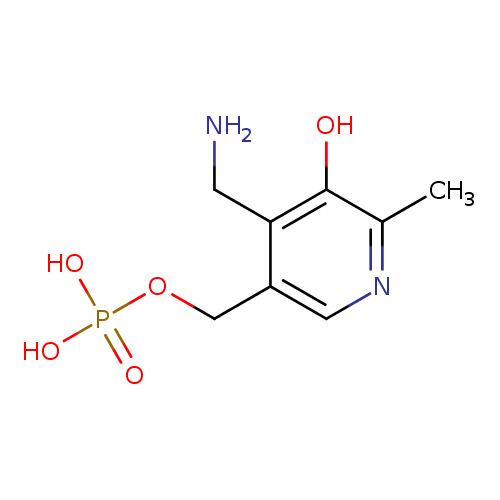
Structure |
|
|---|
| Synonyms: | -
pyridoxamine phosphate
-
PMP
|
|---|
|
Chemical Formula: |
C8H12N2O5P
|
|---|
| Average Molecular Weight: |
247.17 |
|---|
| Monoisotopic Molecular
Weight: |
249.0640330803 |
|---|
| InChI Key: |
ZMJGSOSNSPKHNH-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C8H13N2O5P/c1-5-8(11)7(2-9)6(3-10-5)4-15-16(12,13)14/h3,11H,2,4,9H2,1H3,(H2,12,13,14)/p-1 |
|---|
| CAS
number: |
529-96-4 |
|---|
| IUPAC Name: | [4-(ammoniomethyl)-5-hydroxy-6-methylpyridin-3-yl]methyl phosphate |
|---|
|
Traditional IUPAC Name: |
pyridoxamine-5'-phosphate |
|---|
| SMILES: | CC1(=C(C(=C(COP([O-])([O-])=O)C=N1)C[N+])O) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pyridoxamine 5'-phosphates. These are heterocyclic aromatic compounds containing a pyridoxamine that carries a phosphate group at the 5'-position. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Pyridines and derivatives |
|---|
|
Direct Parent |
Pyridoxamine 5'-phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyridoxamine 5'-phosphate
- Monoalkyl phosphate
- Aralkylamine
- Hydroxypyridine
- Methylpyridine
- Organic phosphoric acid derivative
- Alkyl phosphate
- Phosphoric acid ester
- Heteroaromatic compound
- Azacycle
- Primary aliphatic amine
- Hydrocarbon derivative
- Amine
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Katsunishi, Masatoshi; Kondo, Osamu. Pyridoxamine-5'-phosphate. Jpn. Tokkyo Koho (1972), 2 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|