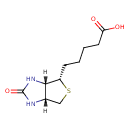
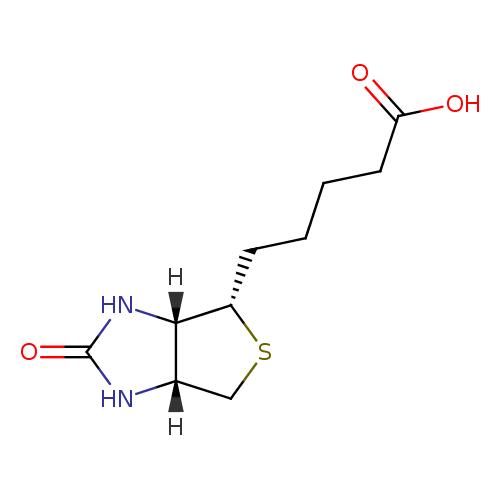
| References: |
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- Thuy LP, Belmont J, Nyhan WL: Prenatal diagnosis and treatment of holocarboxylase synthetase deficiency. Prenat Diagn. 1999 Feb;19(2):108-12. [10215065 ]
- Zempleni J, McCormick DB, Mock DM: Identification of biotin sulfone, bisnorbiotin methyl ketone, and tetranorbiotin-l-sulfoxide in human urine. Am J Clin Nutr. 1997 Feb;65(2):508-11. [9022537 ]
- Bussolati G, Gugliotta P, Volante M, Pace M, Papotti M: Retrieved endogenous biotin: a novel marker and a potential pitfall in diagnostic immunohistochemistry. Histopathology. 1997 Nov;31(5):400-7. [9416479 ]
- Mock DM, Stadler DD, Stratton SL, Mock NI: Biotin status assessed longitudinally in pregnant women. J Nutr. 1997 May;127(5):710-6. [9164991 ]
- Thuy LP, Sweetman L, Nyhan WL: A new immunochemical assay for biotin. Clin Chim Acta. 1991 Oct 31;202(3):191-7. [1814646 ]
- Limat A, Suormala T, Hunziker T, Waelti ER, Braathen LR, Baumgartner R: Proliferation and differentiation of cultured human follicular keratinocytes are not influenced by biotin. Arch Dermatol Res. 1996;288(1):31-8. [8750932 ]
- Bigham SL, Ballard JD, Giles KD, Clelland CS, Jeffcoat R, Griffin KS, Farley TD, Bushman DR, Wright JR: Synthesis and possible applications of biotin-linked copper clusters. Physiol Chem Phys Med NMR. 1990;22(2):63-72. [2100006 ]
- Mock DM, Stadler DD: Conflicting indicators of biotin status from a cross-sectional study of normal pregnancy. J Am Coll Nutr. 1997 Jun;16(3):252-7. [9176832 ]
- Bingham JP, Bian S, Tan ZY, Takacs Z, Moczydlowski E: Synthesis of a biotin derivative of iberiotoxin: binding interactions with streptavidin and the BK Ca2+-activated K+ channel expressed in a human cell line. Bioconjug Chem. 2006 May-Jun;17(3):689-99. [16704206 ]
- Mock DM: Biotin status: which are valid indicators and how do we know? J Nutr. 1999 Feb;129(2S Suppl):498S-503S. [10064317 ]
- Mock DM, Dyken ME: Biotin catabolism is accelerated in adults receiving long-term therapy with anticonvulsants. Neurology. 1997 Nov;49(5):1444-7. [9371938 ]
- Mock DM, Nyalala JO, Raguseo RM: A direct streptavidin-binding assay does not accurately quantitate biotin in human urine. J Nutr. 2001 Aug;131(8):2208-14. [11481419 ]
- Mardach R, Zempleni J, Wolf B, Cannon MJ, Jennings ML, Cress S, Boylan J, Roth S, Cederbaum S, Mock DM: Biotin dependency due to a defect in biotin transport. J Clin Invest. 2002 Jun;109(12):1617-23. [12070309 ]
- Mock DM, Heird GM: Urinary biotin analogs increase in humans during chronic supplementation: the analogs are biotin metabolites. Am J Physiol. 1997 Jan;272(1 Pt 1):E83-5. [9038855 ]
- Fujimoto W, Inaoki M, Fukui T, Inoue Y, Kuhara T: Biotin deficiency in an infant fed with amino acid formula. J Dermatol. 2005 Apr;32(4):256-61. [15863846 ]
- Schenker S, Hu ZQ, Johnson RF, Yang Y, Frosto T, Elliott BD, Henderson GI, Mock DM: Human placental biotin transport: normal characteristics and effect of ethanol. Alcohol Clin Exp Res. 1993 Jun;17(3):566-75. [8333586 ]
- Mock NI, Malik MI, Stumbo PJ, Bishop WP, Mock DM: Increased urinary excretion of 3-hydroxyisovaleric acid and decreased urinary excretion of biotin are sensitive early indicators of decreased biotin status in experimental biotin deficiency. Am J Clin Nutr. 1997 Apr;65(4):951-8. [9094878 ]
- Grafe F, Wohlrab W, Neubert RH, Brandsch M: Transport of biotin in human keratinocytes. J Invest Dermatol. 2003 Mar;120(3):428-33. [12603856 ]
- Gravel RA, Narang MA: Molecular genetics of biotin metabolism: old vitamin, new science. J Nutr Biochem. 2005 Jul;16(7):428-31. [15992684 ]
- Zempleni J: Uptake, localization, and noncarboxylase roles of biotin. Annu Rev Nutr. 2005;25:175-96. [16011464 ]
- Holmberg A, Blomstergren A, Nord O, Lukacs M, Lundeberg J, Uhlen M: The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures. Electrophoresis. 2005 Feb;26(3):501-10. [15690449 ]
|
|---|