|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110768 |
|---|
|
Identification |
|---|
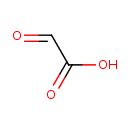
| Name: |
glyoxylate |
|---|
| Description: | An oxo monocarboxylic acid anion in which the oxo group is located at the 2-position. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
glyoxalate
-
glyox
-
glyoxylic acid
|
|---|
|
Chemical Formula: |
C2HO3
|
|---|
| Average Molecular Weight: |
73.028 |
|---|
| Monoisotopic Molecular
Weight: |
74.0003939305 |
|---|
| InChI Key: |
HHLFWLYXYJOTON-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C2H2O3/c3-1-2(4)5/h1H,(H,4,5)/p-1 |
|---|
| CAS
number: |
298-12-4 |
|---|
| IUPAC Name: | 2-oxoacetic acid |
|---|
|
Traditional IUPAC Name: |
glyoxylic acid |
|---|
| SMILES: | [CH](C(=O)[O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as carboxylic acids. These are compounds containing a carboxylic acid group with the formula -C(=O)OH. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Carboxylic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Short-chain aldehyde
- Organooxygen compound
- Carbonyl group
- Aldehyde
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
-93 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | -93 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 1 TMS) | splash10-03di-3900000000-16bc69e0e9d51e54854e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-00di-9000000000-72c34bc34b8c3341442b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-00di-9000000000-920a0dc738957201d4ba | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-00di-9000000000-857c7f2d72c3d4c10dbf | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-00di-9000000000-9b5825d5d9d8b094fefa | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-00di-9000000000-de556f03ea428deff5e2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-00dl-9000000000-74b253632894213d473c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-9000000000-a8cc2c89793394fdf9e4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-9000000000-d20183b08984d4766e8a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-ea9968e3933fd734506c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-9000000000-cef8efc477a2500a7ead | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9000000000-3817c0865df629803538 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9000000000-bb935f857fb5fd08c7e3 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-004l-9000000000-a04bafbf8e0b990094a3 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Graupner M, Xu H, White RH (2000)Identification of an archaeal 2-hydroxy acid dehydrogenase catalyzing reactions involved in coenzyme biosynthesis in methanoarchaea. Journal of bacteriology 182, Pubmed: 10850983
|
|---|
| Synthesis Reference: |
Jie, Yuanping; Song, Zhen. Method for preparing glyoxylic acid. Faming Zhuanli Shenqing Gongkai Shuomingshu (2007), 5pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|