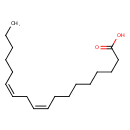
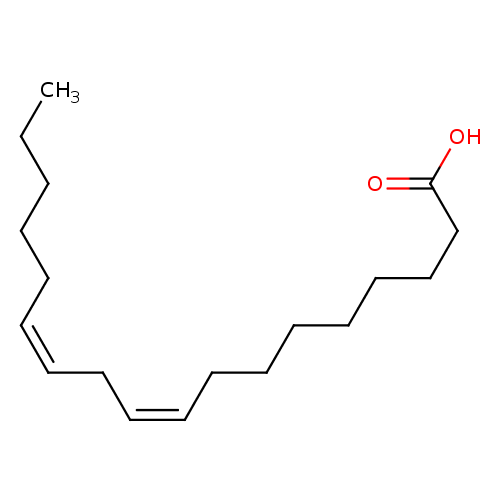
| References: |
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- Hoffmann GF, Meier-Augenstein W, Stockler S, Surtees R, Rating D, Nyhan WL: Physiology and pathophysiology of organic acids in cerebrospinal fluid. J Inherit Metab Dis. 1993;16(4):648-69. [8412012 ]
- Schurer NY, Stremmel W, Grundmann JU, Schliep V, Kleinert H, Bass NM, Williams ML: Evidence for a novel keratinocyte fatty acid uptake mechanism with preference for linoleic acid: comparison of oleic and linoleic acid uptake by cultured human keratinocytes, fibroblasts and a human hepatoma cell line. Biochim Biophys Acta. 1994 Feb 10;1211(1):51-60. [8123682 ]
- Valianpour F, Wanders RJ, Overmars H, Vaz FM, Barth PG, van Gennip AH: Linoleic acid supplementation of Barth syndrome fibroblasts restores cardiolipin levels: implications for treatment. J Lipid Res. 2003 Mar;44(3):560-6. Epub 2002 Dec 16. [12562862 ]
- Horrobin DF: Essential fatty acid metabolism and its modification in atopic eczema. Am J Clin Nutr. 2000 Jan;71(1 Suppl):367S-72S. [10617999 ]
- Imokawa G, Yada Y, Higuchi K, Okuda M, Ohashi Y, Kawamata A: Pseudo-acylceramide with linoleic acid produces selective recovery of diminished cutaneous barrier function in essential fatty acid-deficient rats and has an inhibitory effect on epidermal hyperplasia. J Clin Invest. 1994 Jul;94(1):89-96. [8040295 ]
- Kawajiri H, Hsi LC, Kamitani H, Ikawa H, Geller M, Ward T, Eling TE, Glasgow WC: Arachidonic and linoleic acid metabolism in mouse intestinal tissue: evidence for novel lipoxygenase activity. Arch Biochem Biophys. 2002 Feb 1;398(1):51-60. [11811948 ]
- Iso H, Sato S, Umemura U, Kudo M, Koike K, Kitamura A, Imano H, Okamura T, Naito Y, Shimamoto T: Linoleic acid, other fatty acids, and the risk of stroke. Stroke. 2002 Aug;33(8):2086-93. [12154268 ]
- Seidel D, Heipertz R, Weisner B: Cerebrospinal fluid lipids in demyelinating disease. II. Linoleic acid as an index of impaired blood-CSF barrier. J Neurol. 1980 Jan;222(3):177-82. [6153705 ]
- Salo P, Seppanen-Laakso T, Laakso I, Seppanen R, Niinikoski H, Viikari J, Simell O: Low-saturated fat, low-cholesterol diet in 3-year-old children: effect on intake and composition of trans fatty acids and other fatty acids in serum phospholipid fraction-The STRIP study. Special Turku coronary Risk factor Intervention Project for children. J Pediatr. 2000 Jan;136(1):46-52. [10636973 ]
- Grimsgaard S, Bonaa KH, Jacobsen BK, Bjerve KS: Plasma saturated and linoleic fatty acids are independently associated with blood pressure. Hypertension. 1999 Sep;34(3):478-83. [10489397 ]
|
|---|