|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110738 |
|---|
|
Identification |
|---|
| Name: |
(R)-3-hydroxybutanoate |
|---|
| Description: | The conjugate base of (R)-3-hydroxybutyric acid. |
|---|
|
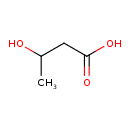
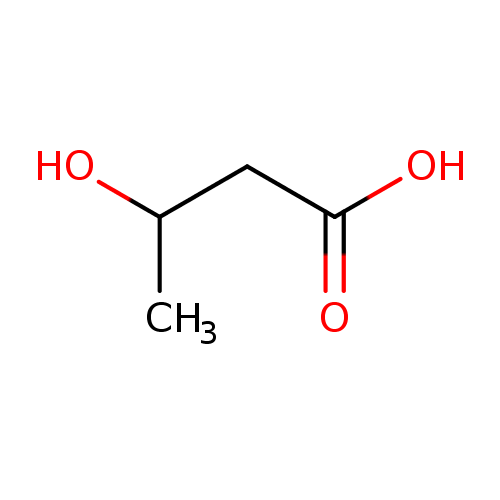
Structure |
|
|---|
| Synonyms: | -
D-3-hydroxybutyrate
-
D-β-hydroxybutyrate
-
(R)-3-hydroxybutanoic acid
-
(R)-3-hydroxybutyric acid
-
β-hydroxybutyric acid
-
b-hydroxybutyric acid
-
(R)-3-hydroxybutyrate
|
|---|
|
Chemical Formula: |
C4H7O3
|
|---|
| Average Molecular Weight: |
103.1 |
|---|
| Monoisotopic Molecular
Weight: |
104.0473441231 |
|---|
| InChI Key: |
WHBMMWSBFZVSSR-GSVOUGTGSA-M |
|---|
| InChI: |
InChI=1S/C4H8O3/c1-3(5)2-4(6)7/h3,5H,2H2,1H3,(H,6,7)/p-1/t3-/m1/s1 |
|---|
| CAS
number: |
300-85-6 |
|---|
| IUPAC Name: | (3R)-3-hydroxybutanoate |
|---|
|
Traditional IUPAC Name: |
3 hydroxybutyrate |
|---|
| SMILES: | CC(CC([O-])=O)O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as beta hydroxy acids and derivatives. These are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
|
Direct Parent |
Beta hydroxy acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Short-chain hydroxy acid
- Beta-hydroxy acid
- Fatty acid
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
- 3-hydroxy fatty acid anion (CHEBI:37054)
- a small molecule (D-3-HYDROXY-BUTYRATE)
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
46 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 46 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 444 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-0002-0900000000-92be5c49a099fe1d9865 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-01ot-1920000000-c93138bf9d35643fef30 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-00dj-8900000000-e632e14fb4be28bdb786 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-014l-1900000000-e192caba63f034040624 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-0a4i-9200000000-4156904e7472b5e97249 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-0a4i-9300000000-505ae46abb0c49c78b1e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-0zfr-9600000000-dfed69c37c1a4d794440 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-0zfr-4900000000-537084557f5986f16b31 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0007-9000000000-7fc414806153ebd8822c | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Tseng HC, Martin CH, Nielsen DR, Prather KL (2009)Metabolic engineering of Escherichia coli for enhanced production of (R)- and (S)-3-hydroxybutyrate. Applied and environmental microbiology 75, Pubmed: 19304817
|
|---|
| Synthesis Reference: |
Yang, Wenling; Ma, Peisheng; Wang, Jianing; Wang, Chunfang; Li, Haixia. Study on the process of chemical synthesis of 3-hydroxybutyric acid. Huaxue Gongcheng (Xi'an, China) (2002), 30(5), 74-78. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|