|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110708 |
|---|
|
Identification |
|---|
| Name: |
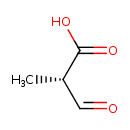
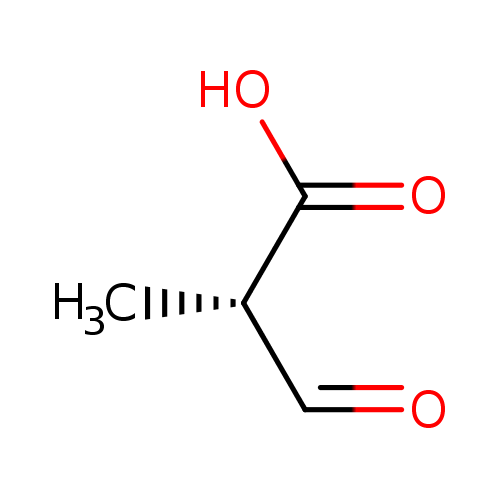
(S)-methylmalonate-semialdehyde |
|---|
| Description: | 2-Methyl-3-oxopropanoate with S configuration at the chiral centre. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
(S)-2-methyl-3-oxopropanoate
-
(S)-ch3-malonate-semialdehyde
|
|---|
|
Chemical Formula: |
C4H5O3
|
|---|
| Average Molecular Weight: |
101.08 |
|---|
| Monoisotopic Molecular
Weight: |
102.0316940589 |
|---|
| InChI Key: |
VOKUMXABRRXHAR-VKHMYHEASA-M |
|---|
| InChI: |
InChI=1S/C4H6O3/c1-3(2-5)4(6)7/h2-3H,1H3,(H,6,7)/p-1/t3-/m0/s1 |
|---|
| CAS
number: |
99043-16-0 |
|---|
| IUPAC Name: | (2S)-2-methyl-3-oxopropanoate |
|---|
|
Traditional IUPAC Name: |
(S)-methylmalonaldehydic acid |
|---|
| SMILES: | CC([CH]=O)C(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 1,3-dicarbonyl compounds. These are carbonyl compounds with the generic formula O=C(R)C(H)C(R')=O, where R and R' can be any group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
1,3-dicarbonyl compounds |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 1,3-dicarbonyl compound
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Hydrocarbon derivative
- Short-chain aldehyde
- Aldehyde
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Propanoate Metabolism pae00640
- Valine, Leucine and Isoleucine Degradation pae00280
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Chambliss KL, Gray RG, Rylance G, Pollitt RJ, Gibson KM: Molecular characterization of methylmalonate semialdehyde dehydrogenase deficiency. J Inherit Metab Dis. 2000 Jul;23(5):497-504. [10947204 ]
- Manning NJ, Pollitt RJ: Tracer studies of the interconversion of R- and S-methylmalonic semialdehydes in man. Biochem J. 1985 Oct 15;231(2):481-4. [4062908 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|