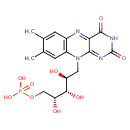
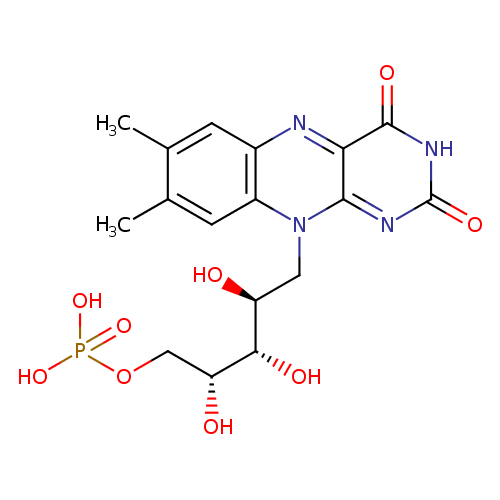
FMN (PAMDB110703)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB110703 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | FMN | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | The trianion arising from deprotonation of the diphosphate hydroxy groups and the imide nitrogen of flavin mononucleotide (FMN). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C17H18N4O9P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 453.32 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 456.1046148038 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | ANKZYBDXHMZBDK-SCRDCRAPSA-K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C17H21N4O9P/c1-7-3-9-10(4-8(7)2)21(15-13(18-9)16(25)20-17(26)19-15)5-11(22)14(24)12(23)6-30-31(27,28)29/h3-4,11-12,14,22-24H,5-6H2,1-2H3,(H3,20,25,26,27,28,29)/p-3/t11-,12+,14-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 146-17-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 1- | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | riboflavin 5'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC2(=CC1(N=C3(C(=O)[N-]C(=O)N=C(N(CC(O)C(O)C(O)COP([O-])(=O)[O-])C=1C=C(C)2)3))) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of chemical entities known as flavin nucleotides. These are nucleotides containing a flavin moiety. Flavin is a compound that contains the tricyclic isoalloxazine ring system, which bears 2 oxo groups at the 2- and 4-positions. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Chemical entities | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Flavin nucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Flavin nucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 290 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 3-(N-morpholino)propanesulfonate + FMNH(2) + Oxygen → 3-(N-morpholino)propanal + Sulfite + Water + FMN + Hydrogen ion 3-hydroxy-9,10-secoandrosta-1,3,5(10)-triene-9,17-dione + Oxygen + FMNH(2) → Hydrogen ion + 3,4-dihydroxy-9,10-secoandrosta-1,3,5(10)-triene-9,17-dione + Water + FMN Butanesulfonate + Oxygen + FMNH(2) → Butanal + Sulfite + Water + FMN + Hydrogen ion FMNH(2) + NAD+ → FMN + NADH + Hydrogen ion isethionate + FMNH(2) + Oxygen → Glycolaldehyde + Sulfite + Water + FMN + Hydrogen ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Ono, Shigeru; Hirano, Hiroko; Sato, Yoshiyuki. Formation of flavin adenine dinucleotide and flavin mononucleotide by lens homogenate. Experimental Eye Research (1982), 34(2), 297-301. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||