|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110685 |
|---|
|
Identification |
|---|
| Name: |
spermidine |
|---|
| Description: | An ammonium ion that is the trication of spermidine, formed by protonation at all three nitrogens. |
|---|
|
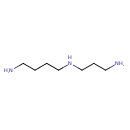
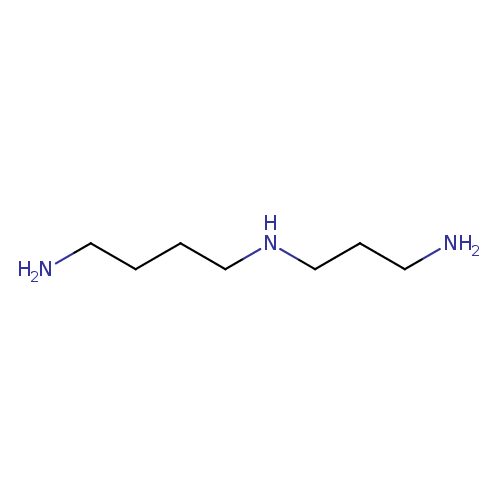
Structure |
|
|---|
| Synonyms: | -
N-(3-aminopropyl)butane-1,4-diamine
|
|---|
|
Chemical Formula: |
C7H22N3
|
|---|
| Average Molecular Weight: |
148.27 |
|---|
| Monoisotopic Molecular
Weight: |
148.1813727218 |
|---|
| InChI Key: |
ATHGHQPFGPMSJY-UHFFFAOYSA-Q |
|---|
| InChI: |
InChI=1S/C7H19N3/c8-4-1-2-6-10-7-3-5-9/h10H,1-9H2/p+3 |
|---|
| CAS
number: |
124-20-9 |
|---|
| IUPAC Name: | (4-azaniumylbutyl)(3-azaniumylpropyl)azanium |
|---|
|
Traditional IUPAC Name: |
spermidine |
|---|
| SMILES: | C([N+])CC[N+]CCCC[N+] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as dialkylamines. These are organic compounds containing a dialkylamine group, characterized by two alkyl groups bonded to the amino nitrogen. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic nitrogen compounds |
|---|
| Sub Class | Organonitrogen compounds |
|---|
|
Direct Parent |
Dialkylamines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Secondary aliphatic amine
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Primary aliphatic amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | +3 |
|---|
|
Melting point: |
< 25 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | < 25 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (5 TMS) | splash10-00di-5910000000-48794a2dc9d682aab28f | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (5 TMS) | splash10-066u-1910000000-9fe6c1c8e8f166270be1 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-00rf-1900000000-c88ed717e55f237cf01c | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (5 TMS) | splash10-00di-6900000000-56396208515d9020bfeb | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (4 TMS) | splash10-0uki-5920000000-4aac8aba8d9635a96f1c | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (5 TMS) | splash10-00rf-1900000000-f4b7d8b055a8d5025036 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (5 TMS) | splash10-00di-1900000000-1be27daaf9f6d9e03752 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00dj-9800000000-02b200749ad6548a5872 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00di-9000000000-c294fdb7876cfc47c089 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00di-9000000000-d3055c2a8763c8034bd2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-052b-0900000000-f08a2a9ba7333f5b725c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-00b9-7900000000-062e21e4525351cbd0d4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-014i-0900000000-31b90f68c9fde4e03f60 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0002-0920000000-28adf399f8ddd0affc22 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0udj-0930000000-3ac88b6d5c40c65f1314 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0006-9000000000-953c054969edecf62533 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-00b9-7900000000-8b03c45141b38c718dac | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-004i-0900000000-1e157eec324aa34dd64e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-0002-0900000000-f2ca6698501cf6a406df | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-00di-9300000000-2f23967aa87f8e5f5bc1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-00di-9000000000-91aef1c8b5a2507a16e9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-00di-9000000000-18cdaecf173b5d79902a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-00di-9000000000-f0b03de09cb13146e794 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-1900000000-300fe40eb32167464ab8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0729-9800000000-1a8be3c6776f3924c8b7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0abc-9000000000-6401e12d220b44018fdf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1900000000-79940496e89b73bb1682 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-3900000000-eb4eaf0bb3e88f274a1d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00du-9000000000-fe762fb3fcd81e27f9c7 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- Venza M, Visalli M, Cicciu D, Teti D: Determination of polyamines in human saliva by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl. 2001 Jun 5;757(1):111-7. [11419735 ]
- Uehara N, Shirakawa S, Uchino H, Saeki Y: Elevated contents of spermidine and spermine in the erythrocytes of cancer patients. Cancer. 1980 Jan 1;45(1):108-11. [7350997 ]
- Proctor MS, Fletcher HV Jr, Shukla JB, Rennert OM: Elevated spermidine and spermine levels in the blood of psoriasis patients. J Invest Dermatol. 1975 Oct;65(4):409-11. [1176793 ]
- El Baze P, Milano G, Verrando P, Renee N, Ortonne JP: Polyamine levels in normal human skin. A comparative study of pure epidermis, pure dermis, and suction blister fluid. Arch Dermatol Res. 1983;275(4):218-21. [6625645 ]
- Mirzoian PA, Promyslov MSh: [Contents of putrescine, spermidine and spermine in tissue of the human brain glial tumors] Ukr Biokhim Zh. 1979 Sep-Oct;51(5):474-6. [516181 ]
- Chaisiri P, Harper ME, Blamey RW, Peeling WB, Griffiths K: Plasma spermidine concentrations in patients with tumours of the breast or prostate or testis. Clin Chim Acta. 1980 Jul 1;104(3):367-75. [6156039 ]
- Martinet N, Beninati S, Nigra TP, Folk JE: N1N8-bis(gamma-glutamyl)spermidine cross-linking in epidermal-cell envelopes. Comparison of cross-link levels in normal and psoriatic cell envelopes. Biochem J. 1990 Oct 15;271(2):305-8. [2241917 ]
|
|---|
| Synthesis Reference: |
Bergeron, Raymond J., Jr. Preparation and formulation spermidine analogues for pharmaceutical use as tumor growth inhibitors. U.S. (2001), 31 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|