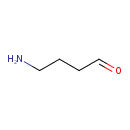
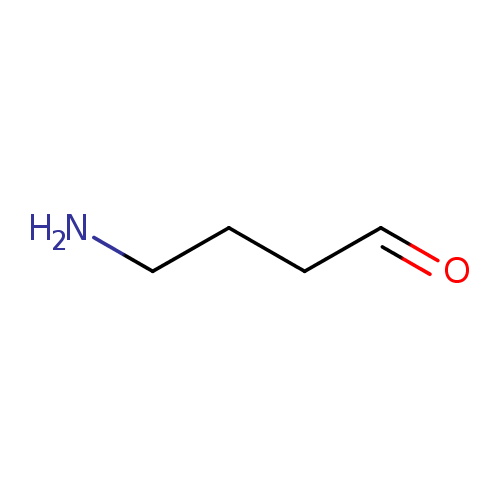
| References: |
- Kikonyogo A, Pietruszko R: Aldehyde dehydrogenase from adult human brain that dehydrogenates gamma-aminobutyraldehyde: purification, characterization, cloning and distribution. Biochem J. 1996 May 15;316 ( Pt 1):317-24. [8645224 ]
- Kurys G, Shah PC, Kikonygo A, Reed D, Ambroziak W, Pietruszko R: Human aldehyde dehydrogenase. cDNA cloning and primary structure of the enzyme that catalyzes dehydrogenation of 4-aminobutyraldehyde. Eur J Biochem. 1993 Dec 1;218(2):311-20. [8269919 ]
- McPherson JD, Wasmuth JJ, Kurys G, Pietruszko R: Human aldehyde dehydrogenase: chromosomal assignment of the gene for the isozyme that metabolizes gamma-aminobutyraldehyde. Hum Genet. 1994 Feb;93(2):211-2. [8112751 ]
- Ambroziak W, Kurys G, Pietruszko R: Aldehyde dehydrogenase (EC 1.2.1.3): comparison of subcellular localization of the third isozyme that dehydrogenates gamma-aminobutyraldehyde in rat, guinea pig and human liver. Comp Biochem Physiol B. 1991;100(2):321-7. [1799975 ]
- Ambroziak W, Pietruszko R: Human aldehyde dehydrogenase: metabolism of putrescine and histamine. Alcohol Clin Exp Res. 1987 Dec;11(6):528-32. [3324802 ]
- Thiele I, Swainston N, Fleming RM, Hoppe A, Sahoo S, Aurich MK, Haraldsdottir H, Mo ML, Rolfsson O, Stobbe MD, Thorleifsson SG, Agren R, Bolling C, Bordel S, Chavali AK, Dobson P, Dunn WB, Endler L, Hala D, Hucka M, Hull D, Jameson D, Jamshidi N, Jonsson JJ, Juty N, Keating S, Nookaew I, Le Novere N, Malys N, Mazein A, Papin JA, Price ND, Selkov E Sr, Sigurdsson MI, Simeonidis E, Sonnenschein N, Smallbone K, Sorokin A, van Beek JH, Weichart D, Goryanin I, Nielsen J, Westerhoff HV, Kell DB, Mendes P, Palsson BO: A community-driven global reconstruction of human metabolism. Nat Biotechnol. 2013 Mar 3. doi: 10.1038/nbt.2488. [23455439 ]
|
|---|