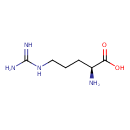
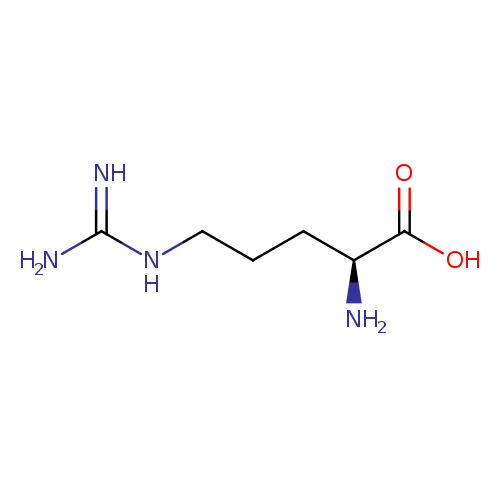
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-0a4i-1910000000-0191c1a63652c493660b | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-9810000000-eb6eb73302b678cf0a24 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-00di-9700000000-e47b41cff0e873f53932 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-0a4i-1920000000-8ae5af11398835d26bed | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00di-3900000000-8c82418f7b35a97fb9b3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00di-9000000000-19b62da79866318c52dd | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00di-9000000000-64b046d21bdcbb8d5923 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-004i-0900000000-e07b937b6867d1f62293 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0a4i-0900000000-87ab853583aab2973cfb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0a4i-2900000000-28814246b728a51e761c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0a4i-2900000000-cfe7e406be2172e94fba | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-004i-0910000000-478a8345915bc15d85b5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0a4i-2900000000-c930c47ecbe6975a00fc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0a4i-2900000000-fa6be0f9ce4ee4c30738 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-004i-0900000000-3425ee9829935182c0d5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0fk9-0946231100-3d57d2304dcb33feab3f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-000i-0900000000-5b6dd6fb263ea09289fc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001i-0900000000-dfe35b3438d19320d8cb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-00di-0900000000-f666ab7e5354bce67a2e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-03kd-0977452210-046c4b70bd0ec351c41d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-014i-9000000000-2f51e43e530976d63633 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001i-0900000000-b9ebb7ebccee1a313888 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-00di-0900000000-5379b6fb6ea2313101f9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - CE-ESI-TOF (CE-system connected to 6210 Time-of-Flight MS, Agilent) , Positive | splash10-004i-0900000000-02b2bc83599dc6944f2e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-00fr-7900000000-b7cd48e0aca6a6256968 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive | splash10-00fr-7900000000-b7cd48e0aca6a6256968 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-00fr-7900000000-4e69a6ce7b97433937ee | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-001i-0900000000-835751d54af24bd337e7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-001i-0900000000-2d2b5fd7617ccb227bb1 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
| References: |
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- Engelborghs S, Marescau B, De Deyn PP: Amino acids and biogenic amines in cerebrospinal fluid of patients with Parkinson's disease. Neurochem Res. 2003 Aug;28(8):1145-50. [12834252 ]
- Hagenfeldt L, Bjerkenstedt L, Edman G, Sedvall G, Wiesel FA: Amino acids in plasma and CSF and monoamine metabolites in CSF: interrelationship in healthy subjects. J Neurochem. 1984 Mar;42(3):833-7. [6198473 ]
- Peng CT, Wu KH, Lan SJ, Tsai JJ, Tsai FJ, Tsai CH: Amino acid concentrations in cerebrospinal fluid in children with acute lymphoblastic leukemia undergoing chemotherapy. Eur J Cancer. 2005 May;41(8):1158-63. Epub 2005 Apr 14. [15911239 ]
- Cynober LA: Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition. 2002 Sep;18(9):761-6. [12297216 ]
- Mizutani N, Hayakawa C, Ohya Y, Watanabe K, Watanabe Y, Mori A: Guanidino compounds in hyperargininemia. Tohoku J Exp Med. 1987 Nov;153(3):197-205. [3433275 ]
- Haas W, Grabe K, Geis C, Pach T, Stoll K, Fuchs M, Haberl B, Loy C: Recognition and invasion of human skin by Schistosoma mansoni cercariae: the key-role of L-arginine. Parasitology. 2002 Feb;124(Pt 2):153-67. [11860033 ]
- Mori A, Watanabe Y, Fujimoto N: Fluorometrical analysis of guanidino compounds in human cerebrospinal fluid. J Neurochem. 1982 Feb;38(2):448-50. [7108550 ]
- Martens-Lobenhoffer J, Bode-Boger SM: Measurement of asymmetric dimethylarginine (ADMA) in human plasma: from liquid chromatography estimation to liquid chromatography-mass spectrometry quantification. Eur J Clin Pharmacol. 2006 Feb;62(Supplement 13):61-68. [16217682 ]
- Stothers L, Laher I, Christ GT: A review of the L-arginine - nitric oxide - guanylate cyclase pathway as a mediator of lower urinary tract physiology and symptoms. Can J Urol. 2003 Oct;10(5):1971-80. [14633324 ]
- Avogaro A, Toffolo G, Kiwanuka E, de Kreutzenberg SV, Tessari P, Cobelli C: L-arginine-nitric oxide kinetics in normal and type 2 diabetic subjects: a stable-labelled 15N arginine approach. Diabetes. 2003 Mar;52(3):795-802. [12606522 ]
- Kotikoski H, Moilanen E, Vapaatalo H, Aine E: Biochemical markers of the L-arginine-nitric oxide pathway in the aqueous humour in glaucoma patients. Acta Ophthalmol Scand. 2002 Apr;80(2):191-5. [11952488 ]
- Noris M, Todeschini M, Cassis P, Pasta F, Cappellini A, Bonazzola S, Macconi D, Maucci R, Porrati F, Benigni A, Picciolo C, Remuzzi G: L-arginine depletion in preeclampsia orients nitric oxide synthase toward oxidant species. Hypertension. 2004 Mar;43(3):614-22. Epub 2004 Jan 26. [14744923 ]
- Kirkeby HJ, Svane D, Poulsen J, Tottrup A, Forman A, Andersson KE: Role of the L-arginine/nitric oxide pathway in relaxation of isolated human penile cavernous tissue and circumflex veins. Acta Physiol Scand. 1993 Nov;149(3):385-92. [8310843 ]
- Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B: Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology. 2000 Dec;119(6):1496-505. [11113071 ]
- Bauchart-Thevret C, Cui L, Wu G, Burrin DG: Arginine-induced stimulation of protein synthesis and survival in IPEC-J2 cells is mediated by mTOR but not nitric oxide. Am J Physiol Endocrinol Metab. 2010 Dec;299(6):E899-909. doi: 10.1152/ajpendo.00068.2010. Epub 2010 Sep 14. [20841502 ]
- Linden KC, Wadley GD, Garnham AP, McConell GK: Effect of l-arginine infusion on glucose disposal during exercise in humans. Med Sci Sports Exerc. 2011 Sep;43(9):1626-34. doi: 10.1249/MSS.0b013e318212a317. [21311355 ]
|
|---|