|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110629 |
|---|
|
Identification |
|---|
| Name: |
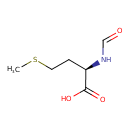
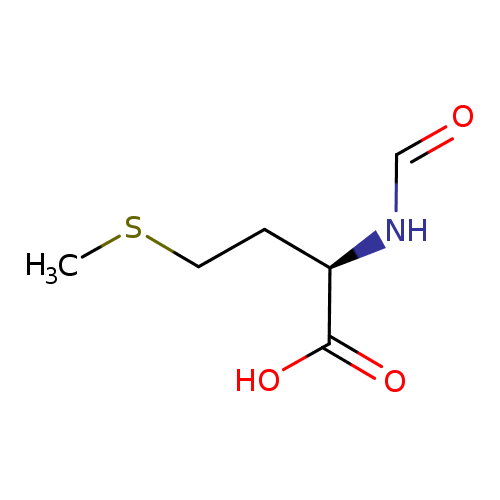
N-formyl-L-methionine |
|---|
| Description: | The conjugate base of N-formyl-L-methionine; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
fMet
-
N-formyl-methionine
-
formylmethionine
|
|---|
|
Chemical Formula: |
C6H10NO3S
|
|---|
| Average Molecular Weight: |
176.21 |
|---|
| Monoisotopic Molecular
Weight: |
177.0459639146 |
|---|
| InChI Key: |
PYUSHNKNPOHWEZ-YFKPBYRVSA-M |
|---|
| InChI: |
InChI=1S/C6H11NO3S/c1-11-3-2-5(6(9)10)7-4-8/h4-5H,2-3H2,1H3,(H,7,8)(H,9,10)/p-1/t5-/m0/s1 |
|---|
| CAS
number: |
4289-98-9 |
|---|
| IUPAC Name: | (2S)-2-formamido-4-(methylsulfanyl)butanoate |
|---|
|
Traditional IUPAC Name: |
N-formylmethionine |
|---|
| SMILES: | C(=O)NC(CCSC)C(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as methionine and derivatives. These are compounds containing methionine or a derivative thereof resulting from reaction of methionine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Methionine and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Methionine or derivatives
- N-formyl-alpha-amino acid
- N-formyl-alpha amino acid or derivatives
- Thia fatty acid
- Fatty acyl
- Fatty acid
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Thioether
- Sulfenyl compound
- Dialkylthioether
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Sherriff RM, Broom MF, Chadwick VS: Isolation and purification of N-formylmethionine aminopeptidase from rat intestine. Biochim Biophys Acta. 1992 Mar 12;1119(3):275-80. [1547272 ]
|
|---|
| Synthesis Reference: |
Milligan D L; Koshland D E Jr The amino terminus of the aspartate chemoreceptor is formylmethionine. The Journal of biological chemistry (1990), 265(8), 4455-60. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|