|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110596 |
|---|
|
Identification |
|---|
| Name: |
D-xylulose 5-phosphate |
|---|
| Description: | An organophosphate oxoanion that is the dianion of D-xylulose 5-phosphate arising from deprotonation of the phosphate OH groups; major species at pH 7.3. |
|---|
|
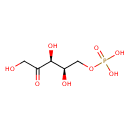
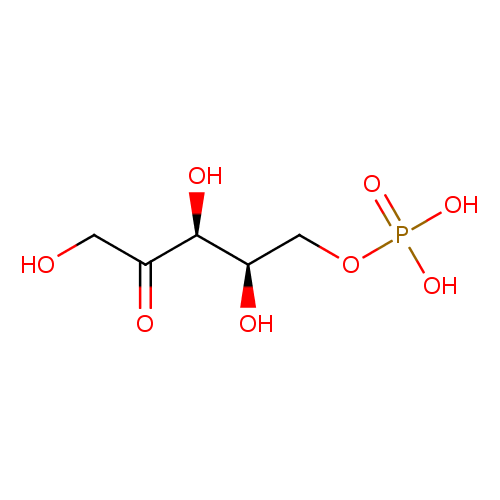
Structure |
|
|---|
| Synonyms: | -
xylulose 5-phosphate
-
xylulose-phosphate
-
D-xylulose-5-P
-
xylulose-P
|
|---|
|
Chemical Formula: |
C5H9O8P
|
|---|
| Average Molecular Weight: |
228.1 |
|---|
| Monoisotopic Molecular
Weight: |
230.0191538399 |
|---|
| InChI Key: |
FNZLKVNUWIIPSJ-RFZPGFLSSA-L |
|---|
| InChI: |
InChI=1S/C5H11O8P/c6-1-3(7)5(9)4(8)2-13-14(10,11)12/h4-6,8-9H,1-2H2,(H2,10,11,12)/p-2/t4-,5-/m1/s1 |
|---|
| CAS
number: |
4212-65-1 |
|---|
| IUPAC Name: | D-threo-pentos-2-ulose 5-phosphate |
|---|
|
Traditional IUPAC Name: |
ribulose-5-phosphate |
|---|
| SMILES: | C(OP([O-])(=O)[O-])C(O)C(O)C(=O)CO |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Pentose phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pentose phosphate
- Pentose-5-phosphate
- Monosaccharide phosphate
- Monoalkyl phosphate
- Acyloin
- Beta-hydroxy ketone
- Organic phosphoric acid derivative
- Alkyl phosphate
- Phosphoric acid ester
- Alpha-hydroxy ketone
- Ketone
- Secondary alcohol
- Polyol
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Alcohol
- Primary alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Nakayama Y, Kinoshita A, Tomita M: Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor Biol Med Model. 2005 May 9;2(1):18. [15882454 ]
- Sanchez B, Champomier-Verges MC, Anglade P, Baraige F, de Los Reyes-Gavilan CG, Margolles A, Zagorec M: Proteomic analysis of global changes in protein expression during bile salt exposure of Bifidobacterium longum NCIMB 8809. J Bacteriol. 2005 Aug;187(16):5799-808. [16077128 ]
- Williams DG: Effect of added xylulose-5-phosphate on the assay of erythrocyte transketolase. Clin Chem. 1977 Jul;23(7):1368. [872397 ]
- Himmo SD, Thomson M, Gubler CJ: Isolation of transketolase from human erythrocytes. Prep Biochem. 1988;18(3):261-76. [3237644 ]
- Veech RL: A humble hexose monophosphate pathway metabolite regulates short- and long-term control of lipogenesis. Proc Natl Acad Sci U S A. 2003 May 13;100(10):5578-80. Epub 2003 Apr 29. [12721358 ]
|
|---|
| Synthesis Reference: |
Shaeri, Jobin; Wohlgemuth, Roland; Woodley, John M. Semiquantitative Process Screening for the Biocatalytic Synthesis of D-Xylulose-5-phosphate. Organic Process Research & Development (2006), 10(3), 605-610. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|